Have you ever wondered who makes the batteries that power our devices? The world of battery technology is evolving rapidly, and two contenders are vying for dominance: aluminum-ion batteries and lithium-ion batteries. This article will explore these two technologies in detail, comparing their features, advantages, disadvantages, and potential applications.
Part 1. What is an aluminum ion battery?
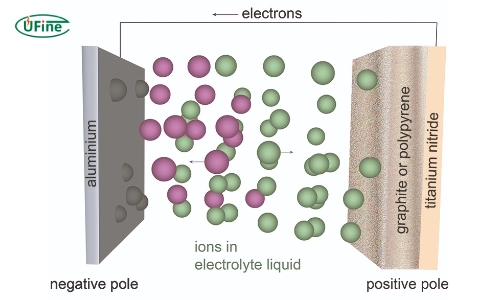
Aluminum ion batteries are rechargeable batteries that use aluminum ions (Al³⁺) as charge carriers. This innovative design allows them to deliver higher energy densities than lithium-ion batteries potentially. Specifically, aluminum can exchange three electrons per ion during charging and discharging. One aluminum ion can carry the equivalent charge of three lithium ions.
The structure of an aluminium ion battery consists of:
- Anode: Made from aluminum.
- Cathode: Typically composed of materials like graphite.
- Electrolyte: Usually an ionic liquid that facilitates the movement of ions between the electrodes.
This configuration enables efficient energy transfer and storage, making aluminum ion batteries a promising alternative to traditional lithium-ion systems.
How do aluminum ion batteries work?
Aluminum ion batteries allow aluminum ions (Al³⁺) to move from the anode to the cathode during discharge and back during charging. This process involves:
- Discharge Phase: Aluminium at the anode oxidizes, releasing Al³⁺ ions into the electrolyte while electrons flow through the external circuit to provide power.
- Charge Phase: Al³⁺ ions migrate back to the anode, which is reduced to metallic aluminum as electrons return from the external circuit.
This mechanism allows for fast charging times—some prototypes can charge in just one minute—and significantly longer cycle lives than traditional lithium-ion batteries.
Part 2. What is a lithium-ion battery?
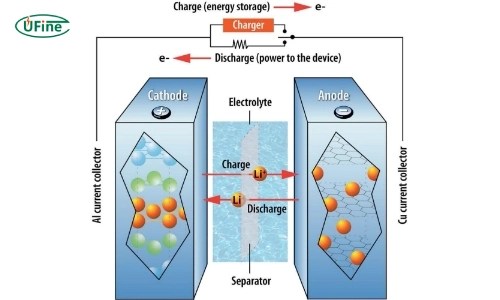
Lithium-ion batteries (Li-ion) are widely used in consumer electronics, electric vehicles, and renewable energy storage systems due to their high energy density and efficiency. These batteries operate on reversible intercalation, where lithium ions move between the anode and cathode during charging and discharging cycles.
Key components of lithium-ion batteries include:
- Anode: Commonly made from graphite.
- Cathode: Composed of various lithium metal oxides.
- Electrolyte: Typically a liquid organic solvent containing lithium salts.
Lithium-ion batteries are known for their:
- High energy density
- Long cycle life (up to 1000 cycles)
- Relatively low self-discharge rates
How do lithium-ion batteries work?
The operation of lithium-ion batteries is based on the movement of lithium ions (Li⁺) between the anode and cathode:
- Discharge Phase: Lithium ions move from the anode (usually graphite) through the electrolyte to the cathode while releasing energy that powers devices.
- Charge Phase: When charging, an external power source drives Li⁺ ions back to the anode, where they intercalate into the graphite structure.
This process is relatively efficient but can lead to dendrite formation—tiny needle-like structures that can cause short circuits and fires if not appropriately managed.
Part 3. What are the main differences between aluminum-ion and lithium-ion batteries?
Several key differences highlight their unique characteristics when comparing aluminum-ion and lithium-ion batteries. Here’s a breakdown of these differences in simple terms:
- Charge Carriers: Aluminium ion batteries use aluminum ions (Al³⁺) as charge carriers, while lithium-ion batteries use lithium ions (Li⁺). This difference is significant as it affects how each battery operates.
- Electron Exchange: Aluminium ions can exchange three electrons per ion during charging and discharging, whereas lithium ions can only exchange one electron per ion. One aluminum ion can carry a charge equivalent to three lithium ions.
- Energy Density: The theoretical energy density of aluminum ion batteries is much higher, reaching up to 1060 Wh/kg, compared to lithium-ion batteries’ maximum of about 406 Wh/kg. This suggests that aluminum ion batteries could store more energy.
- Voltage Output: Aluminium-ion batteries typically have a lower voltage output of about 2.65 V, while lithium-ion batteries operate at around 4 V. This voltage difference can impact the batteries’ overall energy output and efficiency.
- Cycle Life: Aluminium ion batteries have the potential for longer cycle life, with some prototypes showing over 20,000 cycles, whereas lithium-ion batteries generally last around 1000 cycles before their capacity significantly degrades.
- Safety: Aluminium ion batteries are considered safer because they are non-flammable and do not pose the same risks of thermal runaway associated with lithium-ion batteries, which can catch fire if damaged or overheated.
- Material Availability: Aluminium is more abundant and less expensive than lithium, making aluminum ion batteries potentially cheaper to produce on a large scale.
- Environmental Impact: Aluminium is highly recyclable and poses fewer ecological concerns than lithium extraction, which can damage ecosystems.
- Charging Speed: Aluminium-ion batteries can charge significantly faster—up to 60 times quicker than lithium-ion batteries—due to their ability to transfer multiple electrons simultaneously.
- Practical Applications: While aluminum ion technology is still developing, it shows promise for applications requiring fast charging and high safety, such as electric vehicles and grid storage solutions. Lithium-ion technology, on the other hand, is well-established in consumer electronics and electric vehicles.
Comparison Table
| Feature | Aluminium Ion Batteries | Lithium-Ion Batteries |
|---|---|---|
| Charge Carrier | Aluminium ions (Al³⁺) | Lithium ions (Li⁺) |
| Electron Exchange | Three electrons per ion | One electron per ion |
| Energy Density | Up to 1060 Wh/kg | Up to 406 Wh/kg |
| Voltage Output | Approximately 2.65 V | Approximately 4 V |
| Cycle Life | Potentially over 20,000 cycles | Up to 1000 cycles |
| Safety | Non-flammable | Risk of thermal runaway |
| Material Availability | Abundant and cost-effective | Limited resources (lithium mining) |
| Environmental Impact | Highly recyclable | Environmental concerns from mining |
| Charging Speed | Charges up to 60 times faster | Slower charging times |
| Practical Applications | Emerging technology | Established in various industries |
Part 4. Advantages of aluminum ion batteries
Aluminum ion batteries present several notable advantages over their lithium counterparts:
- Fast Charging: They can charge up to 60 times faster than traditional lithium-ion batteries due to their ability to transfer multiple electrons per ion.
- Safety: Aluminium is non-flammable and does not pose the same fire risks associated with lithium-ion technology, making it safer for various applications.
- Environmental Impact: Aluminium is abundant and recyclable, reducing reliance on rare earth metals often used in lithium-ion batteries.
- Cost Efficiency: The materials used in aluminum batteries are generally cheaper than those required for lithium-ion systems.
Part 5. Advantages of lithium-ion batteries
Despite their drawbacks, lithium-ion batteries have established themselves as a reliable technology with several benefits:
- High Energy Density: They provide more energy per unit weight than most other battery types, making them ideal for portable electronics and electric vehicles.
- Mature Technology: Lithium-ion technology has been extensively developed over decades, resulting in robust performance metrics and widespread availability.
- Versatility: They are suitable for various applications, from smartphones to large-scale renewable energy storage systems.
Part 6. Disadvantages of a aluminum on batteries
While promising, aluminum ion batteries also face challenges that hinder their widespread adoption:
- Lower Voltage Output: Currently, they produce lower voltage levels than lithium-ion batteries (approximately 2.65 V vs. around 4 V), limiting their usability in specific applications.
- Development Stage: Many aluminum ion technologies are still in research or prototype stages, lacking commercial viability compared to established lithium-ion products.
Part 7. Disadvantages of lithium-ion batteries
Lithium-ion technology is not without its issues:
- Safety Risks: The potential for thermal runaway can lead to fires or explosions if not properly managed.
- Resource Scarcity: Lithium extraction poses environmental concerns and relies on limited resources found predominantly in specific regions.
- Cycle Life Limitations: While they last around 1000 cycles, this pales compared to some emerging technologies, like aluminum ion, which promise significantly longer lifespans.
Part 8. Applications of aluminum ion vs. lithium-ion batteries
Both types of batteries have distinct applications based on their characteristics:
Aluminium Ion Battery Applications:
- Electric vehicles with fast charging requirements.
- Grid storage solutions for renewable energy due to long cycle life.
- Flexible electronics that require lightweight and bendable power sources.
Lithium-Ion Battery Applications:
- Consumer electronics such as smartphones, laptops, and tablets.
- Electric vehicles with high energy density are crucial for range.
- Renewable energy systems for storing solar or wind power.
Part 9. FAQs
-
What are the main differences between aluminum ion and lithium-ion batteries?
Aluminum ion batteries use Al³⁺ ions, allowing faster charging and better safety than Li⁺ ions used in lithium-ion batteries. -
Is aluminum on batteries safer than lithium-ion?
Aluminum ion batteries are non-flammable and do not pose the same fire risks as lithium-ion technology. -
Can aluminum ion batteries replace lithium-ion in electric vehicles?
While they show promise due to faster charging times and longer cycle life, current-voltage limitations may restrict their immediate use in electric vehicles compared to established lithium-ion systems. -
How long do aluminum ion batteries last?
Aluminium ion batteries can last over 20,000 charge-discharge cycles, significantly outperforming lithium-ion batteries, which typically last around 1,000 cycles. -
What is the environmental impact of using these battery technologies?
Aluminum is more abundant and recyclable than lithium; thus, aluminum ion technology may have a lower overall environmental impact than traditional lithium extraction processes.
Related Tags:
More Articles

How to Choose the Best Floor Scrubber Battery for Commercial Cleaning?
Selecting the ideal floor scrubber battery ensures a long runtime, rapid charging, and minimal maintenance for efficient commercial cleaning operations.
Battery for Blower vs Battery for Leaf Vacuum: Which One Should You Choose?
Battery for blower vs leaf vacuum—learn the key differences in power, fit, and runtime to choose the right battery for your outdoor tool needs.
How to Choose the Right Battery for Blower?
Choosing the right blower battery? Consider voltage, capacity, chemistry & usage. This guide helps match the best battery for peak performance.
How to Choose the Best Insulated Battery Box for Lithium Batteries?
Choosing the Best Insulated Battery Box for Lithium Batteries? Discover key factors such as size, material, and safety for optimal protection and performance.
7 Critical Elements on a Lithium Battery Shipping Label
What must be on a lithium battery shipping label? Learn 7 key elements to ensure safety, legal compliance, and correct handling across all transport modes.