The global battery market is expanding rapidly, driven by innovations in energy storage and increased demand for electric vehicles, portable devices, and renewable energy solutions. According to recent industry statistics, the battery market is expected to grow to $120 billion by 2025, with electric vehicle batteries and renewable energy storage systems leading the charge. As more industries and consumers look to adopt more efficient and sustainable battery solutions, understanding the different types of batteries and their properties is crucial. In this article, we will explore the chemical, physical, and biological battery types, their principles, capacities, voltages, safety aspects, and environmental impact. Whether you’re a developer, manufacturer, or tech enthusiast, this guide will help you make informed decisions about battery technology.
Part 1. What are the different types of batteries?
Batteries can be classified into several categories based on their energy storage mechanisms. The three most prominent categories are chemical, physical, and biological batteries. Each type has its unique characteristics and applications. Let’s break down these categories and explore their principles, structure, and uses.
Part 2. Chemical batteries
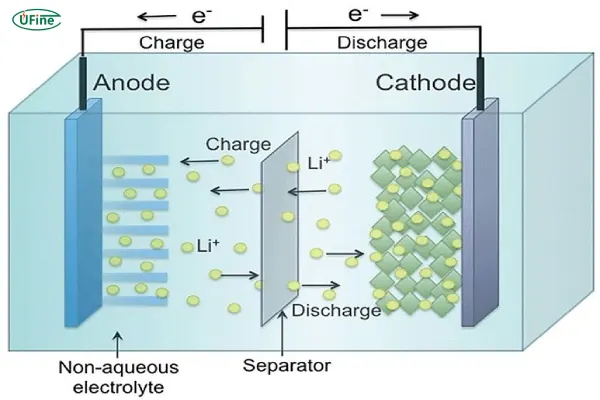
Chemical batteries are the most common type of battery and include lithium-ion batteries, lead-acid batteries, and nickel-metal hydride (NiMH) batteries. These batteries store energy chemically, typically involving the movement of ions between electrodes to create electrical charge.
Principles of Chemical Batteries
Chemical batteries work on the principle of redox reactions, where the battery’s positive and negative electrodes (anode and cathode) facilitate electron flow through an electrolyte. This flow generates the electrical energy needed to power devices.
For example, in lithium-ion batteries, lithium ions move between the cathode and anode during charging and discharging cycles. The energy produced is stable and reliable, making them perfect for electric vehicles, smartphones, and other portable electronics.
Voltage, Capacity, and Structure
The voltage of chemical batteries varies depending on the chemistry used. Lithium-ion batteries, for example, typically have a voltage range between 3.6V to 4.2V per cell. Lead-acid batteries, often used in automotive applications, typically operate at 2V per cell.
Battery capacity is usually measured in ampere-hours (Ah) or milliampere-hours (mAh). Higher values indicate more energy storage. For instance, a lithium-ion battery in an electric vehicle might have a capacity ranging from 30,000 mAh to 100,000 mAh, depending on the application.
Part 3. Physical batteries

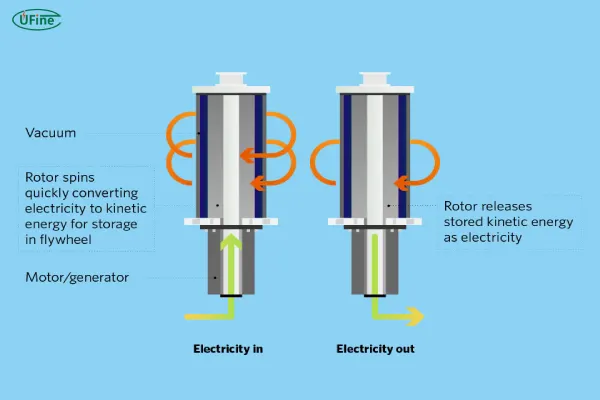
Physical batteries store energy through mechanical processes rather than chemical reactions. The most common example of physical batteries is flywheel energy storage systems. These systems store energy in the form of rotational kinetic energy.
Principles of Physical Batteries
In flywheel-based energy storage, a rotor (flywheel) is spun at high speeds to store mechanical energy. When power is needed, the rotational energy is converted back into electricity. Physical batteries are highly efficient and can provide rapid bursts of power.
Voltage, Capacity, and Structure
The flywheel typically operates at low voltages (10-100V), and its energy capacity depends on the mass of the flywheel and its rotational speed. While the capacity of flywheel systems can be large, they tend to have a shorter discharge time compared to chemical batteries.
Safety and Environmental Impact
Flywheel systems have fewer risks related to thermal runaway and other hazards associated with chemical batteries. They also have a significantly lower environmental impact because they don’t rely on toxic chemicals or materials. However, the efficiency of physical batteries can degrade over time due to mechanical friction.
Part 4. Biological batteries
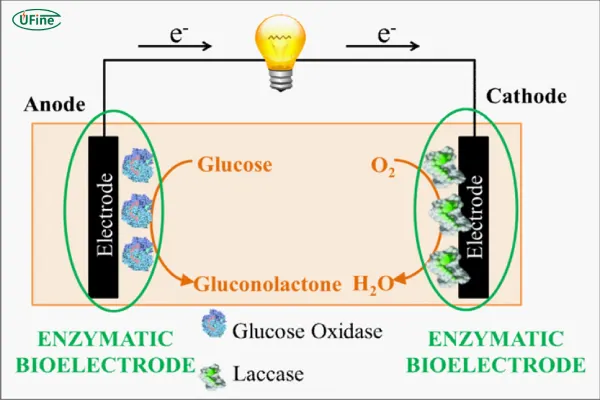
Biological batteries are a relatively new category and are inspired by natural processes, often leveraging microbial fuel cells (MFCs). These systems use living organisms or enzymes to convert organic matter into electrical energy. Biological batteries are still in the research phase for many applications but show great promise in fields like waste treatment and bio-energy.
Principles of Biological Batteries
In microbial fuel cells, microorganisms such as bacteria are used to break down organic matter and produce electrons, which flow through an external circuit, generating electricity. These systems function similarly to chemical batteries, but instead of relying on chemical reactions, they harness biological processes.
Voltage, Capacity, and Structure
The voltage and capacity of biological batteries are relatively low compared to chemical and physical batteries. Typically, microbial fuel cells generate voltages of around 0.2V to 1.0V, making them more suitable for low-power applications or as part of larger, integrated systems.
Safety, Charging, and Cost
Biological batteries have a minimal environmental impact, as they use renewable organic materials, such as waste products. However, they currently face challenges in terms of cost-effectiveness and energy output, which limit their widespread use.
Part 5. How do different battery types compare?
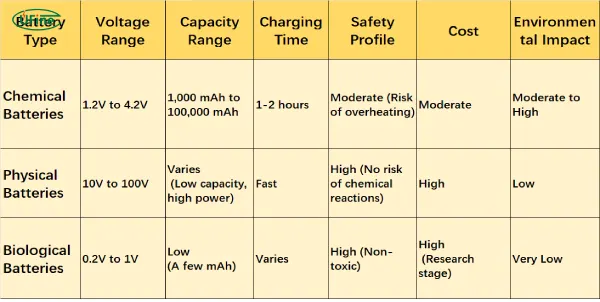
When choosing the right battery for your needs, it’s essential to consider several factors, including voltage, capacity, safety, charging time, cost, and environmental impact. The following comparison table summarizes these aspects for each of the main battery types.
Safety, Cost, and Charging
Chemical batteries generally offer good safety but require proper handling to avoid overheating or short-circuiting. Physical batteries, on the other hand, are inherently safe as they don’t involve chemical reactions, and are increasingly used for high-power applications, like grid storage.
In terms of cost, biological batteries are still in the early stages of development, with a high cost per unit of energy stored. As for charging, chemical batteries have made significant advancements in fast-charging technology, while physical and biological systems may take longer.
Environmental Considerations
Chemical batteries, especially those that rely on lithium-ion chemistry, have a notable environmental footprint due to the mining of materials like lithium and cobalt. However, the impact is relatively lower than that of lead-acid batteries. Biological batteries are considered the most environmentally friendly since they utilize renewable, biodegradable materials.
Part 6. Ufine Battery: custom battery solutions for every need
As the global demand for advanced battery technology increases, Ufine Battery is here to support your specific needs. We are a leading manufacturer of custom lithium-ion batteries, LFP batteries, 18650 cells, and other specialty battery solutions tailored to your unique requirements. Whether you’re developing a new device, or integrating energy storage systems, we can provide the right battery type for your project.
Contact us today to learn more about our high-quality battery materials, including LFP cathodes and other battery components, designed to offer maximum performance, reliability, and safety.
Part 7. Conclusion
In summary, understanding the different types of batteries—chemical, physical, and biological—is crucial for anyone involved in energy storage, renewable energy, or consumer electronics. Each type offers distinct advantages, from the high energy density and efficiency of chemical batteries to the rapid discharge capabilities of physical batteries, and the eco-friendly nature of biological batteries. As battery technology continues to evolve, staying informed will allow you to make smarter choices for your energy storage needs.
Part 8. FAQs
-
What are the key differences between chemical, physical, and biological batteries?
Chemical batteries store energy via chemical reactions between electrodes and electrolytes. Examples include lithium-ion and lead-acid batteries. Physical batteries, like flywheels, store energy in the form of kinetic motion rather than chemical changes. Biological batteries use microbial fuel cells to generate energy through biological processes, such as bacterial reactions to organic matter. -
Which battery type offers the highest energy density?
Chemical batteries, particularly lithium-ion batteries, offer the highest energy density, making them ideal for high-capacity applications like electric vehicles and mobile devices. These batteries can store large amounts of energy in a relatively small space. -
How do flywheel energy storage systems (physical batteries) compare to chemical batteries in terms of charging time?
Flywheel systems (physical batteries) charge much faster than chemical batteries, often in seconds to minutes, because they store energy through rapid rotational motion. In contrast, chemical batteries, such as lithium-ion and lead-acid batteries, typically require 1-2 hours to fully charge, depending on the battery type. -
Are biological batteries commercially available for everyday applications?
While biological batteries, like microbial fuel cells, are still in the research and development phase, they show great promise for low-power applications in sectors like waste treatment and bio-energy. However, their commercial use is limited at the moment due to relatively low energy output and high costs. -
How do environmental impacts compare across different types of batteries?
Biological batteries have the least environmental impact, as they use organic, biodegradable materials and don’t rely on harmful chemicals. Chemical batteries (especially lithium-ion and lead-acid) have a higher environmental footprint due to mining activities and the disposal of toxic materials. Physical batteries like flywheels have a moderate environmental impact, as they do not require chemicals but involve significant manufacturing processes and energy losses during long-term operation.
Related Tags:
More Articles
Paper Battery vs. Flexible Battery: What’s the Difference and Which Is Better?
Paper vs. flexible batteries: learn the key differences, benefits, and which power source fits best for wearables, sensors, and smart tech.
What to Know Before Buying a Tiny LiPo Battery for Your Project
Tiny LiPo batteries are powerful and compact. Learn how to choose the right one for your project with specs, safety, and charging tips.
Bloated LiPo Battery: Will It Explode?
Will a bloated LiPo battery explode? Discover the causes, risks, safety steps, and expert tips to avoid disaster and protect your gear. Must-read safety guide!
12V 100Ah Lithium Ion Battery Price: Full Guide
Learn about 12V 100Ah lithium-ion battery price, from cost ranges to best brands, hidden fees, and how to get the best deal. A must-read for smart buyers!
Resistance and Conductivity: What It Means for Your Lithium Batteries
Resistance and conductivity impact lithium battery performance, lifespan, and safety—learn how they work and why they matter.