- Part 1. How is lithium iron phosphate made?
- Part 2. How preparation methods affect LiFePO4 performance
- Part 3. Applications
- Part 4. Challenges and future directions for LiFePO4
- Part 5. Global situation of lithium iron phosphate materials
- Part 6. Market price of lithium iron phosphate
- Part 7. Challenges and opportunities
- Part 8. Summary and outlook
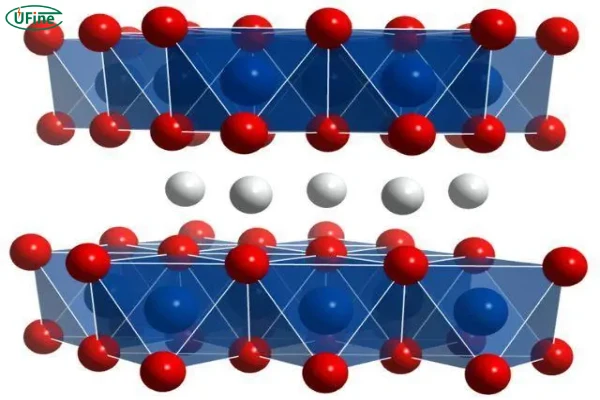
Lithium iron phosphate (LiFePO4) is a critical cathode material for lithium-ion batteries. Its high theoretical capacity, low production cost, excellent cycling performance, and environmental friendliness make it a focus of research in the field of power batteries.
Globally, researchers are working to enhance the specific capacity of LiFePO4, employing methods such as doping and surface coating to optimize its performance. This article provides an overview of LiFePO4 preparation methods, highlights recent advancements, addresses challenges, and explores its potential future development.
Part 1. How is lithium iron phosphate made?
LiFePO4 can be synthesized using methods like solid-state reaction, co-precipitation, and sol-gel processes.
1. Solid-State Reaction Method
This involves reacting transition metal salts (e.g., Fe³⁺, Fe²⁺) with lithium phosphate (Li₄PO₄) under high temperatures, resulting in LiFePO4 precipitation.
2. Co-Precipitation Method
In this approach, precursors such as Fe(NO₃)₃·9H₂O, Li₂CO₃, and lithium-containing phosphates are dissolved in deionized water. After mixing, the precipitates are filtered, washed, dried, and processed into LiFePO4. This method is valued for its simplicity, low raw material cost, and minimal equipment requirements.
3. Sol-Gel Method
Using organic precursors, this technique forms a gel-like substance through reactions at controlled temperatures. After drying and calcination, the LiFePO4 material is obtained. This method offers broad precursor availability and low cost but is sensitive to impurities that may degrade performance.
Part 2. How preparation methods affect LiFePO4 performance
Two primary preparation methods dominate: high-temperature solid-state synthesis and thermal decomposition.
-
High-Temperature Solid-State Synthesis
- Pros: High product purity, uniform particle distribution, and stable crystalline structure.
- Cons: Large particle size, long reaction times, and stringent synthesis conditions increase costs.
-
Thermal Decomposition
- Pros: Produces smaller, narrowly distributed particles with good crystalline properties.
- Cons: Impurities, lower crystallinity, and suboptimal morphology.
While both methods produce usable LiFePO4, the solid-state method is preferred for industrial production due to better control over particle size and shape, contributing to lower costs.
Part 3. Applications
Lithium-ion batteries are categorized based on their cathode materials, with LiFePO4 gaining attention for its safety, environmental benefits, and cost-effectiveness. Its applications include:
- Electric Vehicles (EVs): Widely adopted for their safety and longevity.
- Electric Bicycles and Portable Electronics: Popular for their balance of performance and affordability.
- Energy Storage: Used in power grids and renewable energy storage systems due to stable cycling performance.
Compared to other cathode materials, LiFePO4 offers several advantages:
- Low cost.
- Non-toxicity.
- High safety and cycling stability.
However, challenges remain, including high production costs and potential microcracks during cycling that shorten battery lifespan.
Part 4. Challenges and future directions for LiFePO4
Despite its advantages, LiFePO4 has limitations that must be addressed for broader adoption.
1. Improving Synthesis Techniques
- Solid-state methods result in coarse particles prone to aggregation, reducing performance.
- Liquid-phase reactions can produce uncontrollable morphologies and byproducts.
2. Enhancing Doping Modifications
Doping improves capacity and cycling performance but may also cause structural defects or larger particle sizes if not optimized.
3. Reducing Production Costs
Although raw materials for LiFePO4 are relatively affordable, key components like iron powders remain expensive. Lowering costs is critical for wider application.
4. Boosting Stability
LiFePO4 performs well at room temperature but struggles in high-temperature or high-humidity environments. Composite materials and advanced coatings can improve thermal and electrochemical stability.
Part 5. Global situation of lithium iron phosphate materials
Lithium iron phosphate is at the forefront of research and development in the global battery industry. Its importance is underscored by its dominant role in the production of batteries for electric vehicles (EVs), renewable energy storage systems, and portable electronic devices.
-
China’s Leadership in LFP Production
China is the largest producer and consumer of lithium iron phosphate materials. Its dominance in the battery manufacturing sector, coupled with government policies promoting renewable energy and EV adoption, has cemented its position as the global leader in LFP production. Major manufacturers like CATL and BYD have scaled up LFP battery production to meet the growing demand for cost-effective, long-lasting batteries. -
Emergence of LFP in Western Markets
In the West, the adoption of LFP materials has been slower but is rapidly gaining traction. Companies like Tesla have started integrating LFP batteries into their vehicle lineup, particularly for standard-range models. The shift is driven by the material’s inherent safety, reduced cost compared to nickel-rich cathode materials, and its superior thermal stability. -
Challenges in Supply Chain and Raw Materials
The production of lithium iron phosphate relies on critical raw materials, including lithium, iron, and phosphate. While iron and phosphate are relatively abundant, the sourcing of lithium has become a bottleneck due to the increasing demand from various industries. Geopolitical tensions, environmental concerns, and the limited number of lithium mining facilities have added complexity to the supply chain. -
R&D Efforts Across the Globe
Researchers globally are working to improve LFP’s energy density, enhance its conductivity, and reduce production costs. Universities and private companies are exploring modifications such as doping, surface coatings, and structural optimizations to make the material even more competitive against alternatives like nickel-cobalt-manganese (NCM) and nickel-cobalt-aluminum (NCA) cathodes.
Part 6. Market price of lithium iron phosphate
The market price of lithium iron phosphate materials fluctuates due to factors like raw material costs, production efficiency, and market demand. As of recent years, the price of LFP has been relatively stable compared to other battery materials, making it an attractive choice for large-scale applications.
-
Current Pricing Trends
On average, the price of LFP cathode materials ranges between $6,000 to $10,000 per ton, depending on quality and supplier. This is significantly lower than the cost of nickel or cobalt-based cathode materials, which can exceed $30,000 per ton. -
Impact of Lithium Prices
Lithium carbonate, a key ingredient in LFP production, has experienced price surges due to high demand. This has slightly increased LFP production costs in recent years. However, the availability of alternative lithium sources and innovations in recycling lithium from used batteries are expected to stabilize costs in the future. -
Economic Viability in Applications
The relatively low price of LFP materials has made them a preferred choice for utility-scale energy storage systems and EVs, especially in price-sensitive markets. Coupled with their longer lifespan and high safety standards, LFP batteries offer a cost-effective solution for meeting global energy needs.
Part 7. Challenges and opportunities
Despite its numerous advantages, lithium iron phosphate faces challenges that need to be addressed for wider adoption:
- Energy Density: LFP batteries have a lower energy density compared to NCM or NCA batteries, which limits their use in applications requiring high energy storage in a compact form.
- Recycling and Disposal: Although LFP is environmentally friendly, efficient recycling methods are necessary to handle large-scale adoption and ensure sustainability.
- Raw Material Sourcing: Ensuring a steady supply of lithium while addressing environmental and geopolitical concerns is crucial for the material’s growth.
On the opportunity side, advancements in solid-state batteries and hybrid chemistries could further enhance the performance and appeal of lithium iron phosphate materials. The global push toward renewable energy and green technologies ensures that LFP will remain a vital player in the energy landscape for decades to come.
Part 8. Summary and outlook
LiFePO4 stands out as a high-energy-density cathode material with advantages such as safety, long lifespan, and environmental friendliness. However, its high-temperature performance and production costs limit its application to areas like low-speed electric vehicles and energy storage.
Modification techniques, including doping and surface coatings, show promise but introduce trade-offs between cost and performance. Continued research is essential to develop cost-effective methods and improve material quality.
As the demand for EVs and renewable energy storage grows, LiFePO4’s role in the lithium-ion battery market will likely expand, driving innovation and overcoming current limitations.
Related Tags:
More Articles

How Long Do Rechargeable AA Batteries Last?
How long do rechargeable AA batteries last? Compare NiMH and lithium AA lifespan, recharge cycles, key factors, and performance vs alkaline batteries.
12V STD vs 12V AGM: Meaning, Differences, and Which Is Better
Understand what STD and AGM batteries mean, their key differences, and which 12V battery fits your needs best in 2026.
Battery Reconditioning Explained: A Comprehensive Guide
Learn what battery reconditioning is, how it works, how long it takes, and when reconditioning chargers are used for lead-acid and lithium-ion batteries.
Recommended 10 Best Batteries For Smoke Detectors
Compare the best batteries for smoke detectors in 2026. See which 9V lithium and alkaline batteries last longest and perform best in smoke alarms.
Triple A Battery Voltage: Everything You Need to Know
How many volts is a AAA battery? See a clear AAA battery voltage chart, compare alkaline, lithium and NiMH cells, and learn how voltage affects device use.