- Part 1. What are sodium-ion batteries?
- Part 2. What are lithium-ion batteries?
- Part 3. Comparing sodium-ion and lithium-ion batteries
- Part 4. Sodium-ion battery vs lithium-ion battery: current applications
- Part 5. Future developments in sodium-ion technology
- Part 6. Challenges facing sodium-ion batteries
- Part 7. FAQs
Sodium-ion batteries are emerging as a potential alternative to lithium-ion batteries, which dominate the current energy storage market. As the demand for sustainable and efficient energy solutions grows, understanding the capabilities and limitations of sodium-ion batteries becomes increasingly essential. This article explores the fundamentals of battery technologies, their comparative advantages and disadvantages, and the future of energy storage.
Part 1. What are sodium-ion batteries?
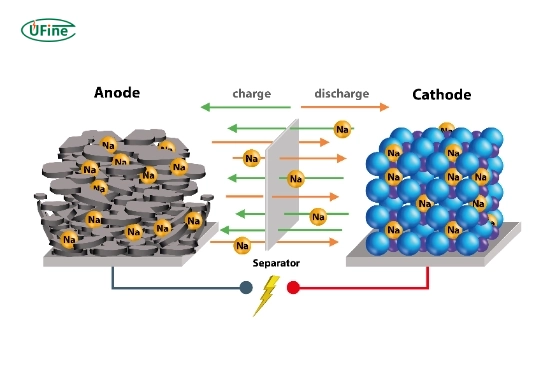
Sodium-ion batteries (NIBs) are rechargeable batteries that utilize sodium ions as the primary charge carrier. Their basic principle mirrors that of lithium-ion batteries, where ions move between the anode and cathode during charging and discharging cycles.
Key Components of Sodium-Ion Batteries
- Anode: The anode in sodium-ion batteries is often made from materials like hard carbon or sodium titanate. These materials allow for efficient sodium ion intercalation, which is crucial for battery performance.
- Cathode: The cathode typically consists of layered oxides, polyanionic compounds, or Prussian blue analogs. These materials are designed to host sodium ions during the discharge cycle.
- Electrolyte: The electrolyte is a sodium salt dissolved in a solvent that facilitates the movement of sodium ions between the anode and cathode.
How Do Sodium-Ion Batteries Work?
When a sodium-ion battery charges, sodium ions migrate from the cathode to the anode through the electrolyte. During discharge, these ions return to the cathode, releasing energy that can be used to power devices. This movement of ions enables the battery to store and release energy efficiently.
Part 2. What are lithium-ion batteries?
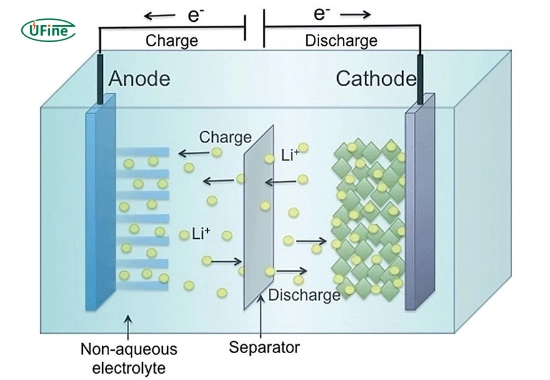
Lithium-ion batteries (LIBs) have become the standard for energy storage in various applications due to their high energy density and efficiency. They operate similarly to sodium-ion batteries but utilize lithium ions instead.
Key Components of Lithium-Ion Batteries
- Anode: Typically constructed from graphite or silicon-based materials, which allow lithium ions to intercalate effectively during charging.
- Cathode: Often made from lithium cobalt oxide or lithium iron phosphate, these materials provide a suitable environment for lithium-ion storage.
- Electrolyte: A lithium salt dissolved in an organic solvent, allowing efficient ion transport between the anode and cathode.
How Do Lithium-Ion Batteries Work?
In lithium-ion batteries, lithium ions move from the anode to the cathode during charging and back again during discharging. This process enables efficient energy storage and release, making them ideal for applications ranging from smartphones to electric vehicles.
Part 3. Comparing sodium-ion and lithium-ion batteries
When evaluating whether sodium-ion batteries can replace lithium-ion batteries, it is essential to compare their performance across several key metrics:
Energy Density
- Lithium-ion batteries usually have an energy density of 150 to 250 Wh/kg. This makes them ideal for applications that need high energy output.
- Sodium-ion batteries have a lower energy density, around 100 to 150 Wh/kg. This limit may make them less suitable for applications where space and weight matter.
Cost Considerations
- Material Availability: Lithium comes from limited sources like brine pools and hard rock mines, which causes price changes. In contrast, sodium is abundant and cheap, mainly sourced from seawater and salt deposits.
- Production Costs: The lower cost of sodium’s raw materials could lead to cheaper production compared to lithium-ion batteries.
Environmental Impact
- Lithium Mining Concerns: Extracting lithium can cause significant harm to the environment, such as water depletion and habitat destruction.
- Sodium’s Environmental Footprint: Sodium is more eco-friendly due to its wide availability and lower extraction impact. Sodium-ion batteries may also be easier to recycle than lithium-ion batteries.
Performance Characteristics
- Charge/Discharge Rates: Lithium-ion batteries are known for fast charging. Sodium-ion batteries charge and discharge more slowly but are improving with new materials.
- Cycle Life: Both battery types can last for many cycles. Recent advancements in sodium-ion technology are helping to close this gap.
| Feature | Lithium-Ion Batteries | Sodium-Ion Batteries |
|---|---|---|
| Energy Density | 150 – 250 Wh/kg | 100 – 150 Wh/kg |
| Material Cost | Higher due to limited sources | Lower due to abundant sources |
| Environmental Impact | Significant concerns | Lower impact |
| Charge/Discharge Rates | Fast | Slower but improving |
| Cycle Life | Long-lasting | Improving |
Part 4. Sodium-ion battery vs lithium-ion battery: current applications
Understanding where each battery type is currently utilized provides insight into their strengths.
Applications of Lithium-ion Batteries
- Consumer electronics (smartphones, laptops)
- Electric vehicles
- Renewable energy storage systems
Applications of Sodium-ion Batteries
Currently limited but expanding into:
- Grid storage solutions
- Low-cost electric vehicles
- Renewable energy integration
Part 5. Future developments in sodium-ion technology
Research into sodium-ion technology is rapidly advancing. It focuses on enhancing performance metrics such as energy density and cycle life.
Innovative Materials
New materials like sodium manganese oxide and advanced carbon composites show promise for improving battery performance.
Potential Market Growth
As demand for sustainable energy solutions increases, sodium-ion batteries may be more prominent in various industries.
Part 6. Challenges facing sodium-ion batteries
Despite their potential advantages, several challenges must be addressed before sodium-ion batteries can compete effectively with lithium-ion technologies.
Technical Limitations
Current limitations include:
- Lower energy density
- Slower charge/discharge rates
- Limited commercial production capabilities
Market Acceptance
- Transitioning industries from established lithium technologies to emerging sodium technologies requires overcoming skepticism regarding performance reliability and longevity.
Part 7. FAQs
-
What are the main advantages of sodium-ion batteries?
Sodium-ion batteries are cheaper due to abundant raw materials and have a lower environmental impact than lithium-ion batteries. -
Can sodium-ion batteries match the performance of lithium-ion batteries?
They need to catch up in energy density, but ongoing research aims to improve their performance metrics significantly. -
Are sodium-ion batteries safer than lithium-ion batteries?
Sodium-ion batteries are less prone to thermal runaway reactions than their lithium-ion counterparts, potentially making them safer under certain conditions. -
What industries could benefit most from sodium-ion technology?
Industries focused on grid storage solutions and low-cost electric vehicles may benefit significantly from adopting sodium-ion technology. -
How long will it take for sodium-ion batteries to become mainstream?
While advancements are promising, widespread adoption may take several years as research continues and production scales up.
Related Tags:
More Articles

What are Watts and Watt Hours in Battery?
Understand watt vs watt-hour in batteries, how to calculate battery watt hours, and what Wh means for car batteries, devices, and energy storage.
A Complete Guide to the Best Batteries for Flashlights
Compare the best batteries for flashlights, including AA, AAA, 18650, 21700, CR123A. See which battery offers the best brightness, runtime, and reliability.
How Long Do Rechargeable AA Batteries Last?
How long do rechargeable AA batteries last? Compare NiMH and lithium AA lifespan, recharge cycles, key factors, and performance vs alkaline batteries.
How Much Current Can a 9V Battery Really Supply?
Discover how many amps a 9V battery can supply, its actual current output, discharge rate, and capacity for alkaline, lithium, and rechargeable 9V batteries.
12V STD vs 12V AGM: Meaning, Differences, and Which Is Better
Understand what STD and AGM batteries mean, their key differences, and which 12V battery fits your needs best in 2026.