The world constantly leads towards sustainable electrification solutions that majorly deploy clean energy technologies. With time, their demand is rapidly increasing. Lithium-ion batteries are playing a critical role in this race. From our smartphones to electric vehicles, we are using them to power almost everything. So, it is important to understand how these batteries work. Particularly, we must know about the major components, i.e., the cathode and anode of the battery. It will ultimately help us get deep knowledge of how these power sources work.
It has two important components, anode and cathode, which help define the battery’s performance and specification in detail.
So, if you are new and do not know much about these components, you are at the right place. This article will help you learn about the definition of cathode and anode of battery. We will discuss, i.e., lithium-ion battery material, the working process, and their roles in promoting clean energy.
Part 1. Anode and cathode definition
If you are a beginner and want to know what an anode and cathode are, you are at the right place. So, here is their description.
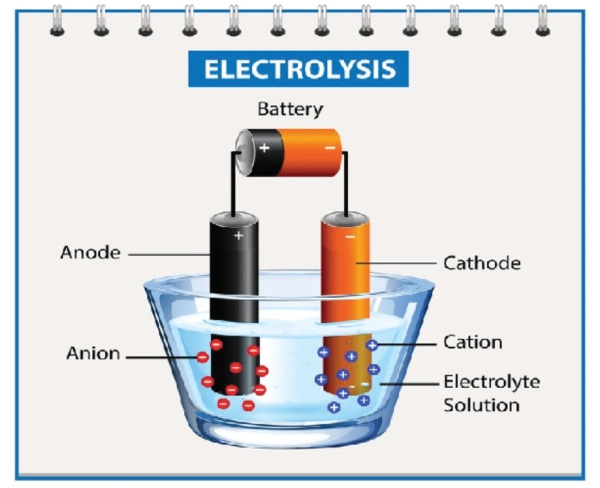
Cathodes and Anodes are electrodes of any battery or electrochemical cell. These help in the flow of electrical charges inside the battery. Moreover, the cathode has a positive charge, where reduction occurs (receives electrons). In contrast, the anode has a negative charge, where oxidation occurs (loss of electrons) and electricity is produced. On the other hand, if we talk about the charging process of a battery, electrons flow from the cathode to the anode (reverse order), storing energy that can later be used to power devices.
So, the following is a description to help you understand the anode and cathode and how they work in any battery or electrochemical cell.
What is a Battery Anode?
The anode is one of the essential components of the battery. It is a negative electrode which is immersed in an electrolyte solution. So, when the current is allowed to pass through the battery, it oxidizes itself, and the negative charges start to lose and travel towards the positive electrode.
What is the Battery Cathode?
In contrast to the anode, the cathode is a positive electrode of the battery. It gets electrons and is reduced itself. Moreover, the cathode is immersed in the battery’s electrolyte solution. So, when the current is allowed to pass, the negative charges move from the anode side and reach the cathode. The cathode gains these negatively charged electrons. Thus, it reduces itself. This constant flow of negative charges towards positive ones helps generate electricity via batteries. Electrolyte solution is bridging in transmitting electrons from one electrode to another.
Part 2. Battery anode vs cathode: What’s the difference?
The cathode and anode of the battery are of different types. Thus, they serve for a different and unique purpose. So, here is a detailed aspect of how the anode and cathode and anode of a battery have different natures from each other;
Table 1: Difference between Battery Anode and Cathode
| Aspect | Anode | Cathode |
|---|---|---|
| Direction of Current | Current flows into the anode | Current flows out of the cathode |
| Electron Flow | Electrons are released (oxidation) | Electrons are gained (reduction) |
| Charge | Typically considered negative | Usually labelled as positive |
| Chemical Reactions | Site of oxidation reactions | Site of reduction reactions |
| Mass Changes | May undergo changes in mass | Generally, experiences minimal mass changes |
| Material Composition | Often graphite or lithium-based materials | Often metal oxides or lithium-based materials |
| Function in Battery | Supplies electrons to the external circuit during discharge | Accepts electrons from the external circuit during discharge |
| Ion Movement | Attracts positively charged ions (cations) | Attracts negatively charged ions (anions) |
| State of Charge | Typically depleted during discharge | Typically enriched during discharge |
| Potential Difference | Generally, has a lower potential relative to the cathode | Generally, has a higher potential relative to the anode |
| Reactivity | Tends to be more reactive | Tends to be less reactive |
| Conductivity | Typically, higher electrical conductivity | Typically, lower electrical conductivity |
Part 3. Battery positive and negative Electrodes
Batteries are also known as secondary cells. In 2019, the Nobel Chemistry Prize was given for developing Lithium-Ion Batteries. Since then, we have witnessed significant development in rechargeable batteries.
When people talk about battery electrodes, they often confuse the terms anode, cathode, positive and negative electrode. They think these refer to each other, which is not true and can lead to errors. So, here is a clear definition of positive and negative electrode;
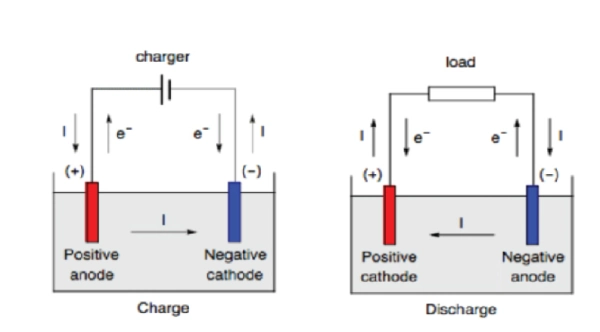
At the Anode, an oxidation reaction occurs: the loss of electrons. A reduction reaction occurs at the cathode, which is a gain of electrons for the electroactive species. But if we deal with batteries, oxidation and reduction sometimes occur at the same electrode. This can occur during charging or discharging. So, it is important to refer to electrodes with positive or negative electrodes instead of cathode and anode.
Is an anode negative or positive?
The positive electrode has a higher potential than the negative electrode. So, when the battery discharges, the cathode acts as a positive, and the anode is negative.
Is the cathode negative or positive?
Similarly, during the charging of the battery, the anode is considered a positive electrode. At the same time, the cathode is called a negative electrode.
Part 4. Battery positive vs negative: What’s the difference?
For a better understanding, we summarise the concept of negative and positive electrodes for batteries in the following table.
Table 2: Difference Between the battery positive and negative electrodes
| Aspect | Positive Electrode | Negative Electrode |
|---|---|---|
| Location during Discharge | Cathode | Anode |
| Location during Charging | Anode | Cathode |
| Electrochemical Reaction | Reduction reaction (gain of electrons) | Oxidation reaction (loss of electrons) |
| Charge | Positive | Negative |
| Potential | Higher potential relative to the negative electrode | Lower potential relative to the positive electrode |
| Function in Battery | Supplies electrons to the external circuit during discharge | Accepts electrons from the external circuit during discharge |
| Ion Movement | Attracts positively charged ions (cations) | Attracts negatively charged ions (anions) |
| State of Charge | Enriched during discharge | Depleted during discharge |
| Conductivity | Lower electrical conductivity | Higher electrical conductivity |
Part 5. Lithium ion batteries material

Lithium-ion batteries can flow the right amount of charge. This is why they have flawless components, i.e., Anode and Cathode material. As battery technology emerges, the anode and cathode materials play a significant role. So, let’s discuss the differences between materials used in the anode and cathode of Lithium-Ion batteries.
What Is Lithium-ion Battery Cathode?
Lithium-ion cathode stores and releases the lithium ions during the charging and discharging of the battery. It is a positive electrode and undergoes a reduction reaction during discharge. Hence, the lithium-ions are captured within the structure.
What Is Lithium-ion Battery Cathode Materials?
When we study the complete working of cathodes, we see that their manufacturing material also plays a crucial role. Because they help estimate the characteristics and overall performance of the battery. So, here is an overview of some commonly used cathode materials in Lithium-ion Batteries.
- Lithium Cobalt Oxide (LiCoO2) is generally used in commercial Li-Ion batteries. Because they have high energy density and stable cycling performance; however, cobalt is an expensive material and is less available. So, research is being carried out to find alternative materials for cathodes.
- Lithium Iron Phosphate (LiFePO4): Regarding vehicles, they also require batteries. So, LiFePO4 is considered a popular choice in this aspect. The reason for its preference is it is safe and quite stable. Moreover, it has less energy density than Lithium Cobalt Oxide (LiCoO2). But it has a longer cycle of life and better thermal stability.
- Lithium Nickel Manganese Cobalt Oxide (NMC) :It is also quite popular among electric vehicles and portable electronic devices. So, MNC has balanced thermal stability, high energy density, and a long life cycle.
- Lithium Nickel Cobalt Aluminum Oxide (NCA): Just like NMC, NCA is also considered a good fit for high-performance applications i.e., grid storage systems and electric vehicles.
- Layered Oxides (LMO) and Spinel Oxides: This material belongs to the class of layered oxides cathodes. Hence, they have high energy density. Moreover, it has a good rate capability, making it suitable for several applications.
What Is Lithium-ion Battery Anode?
While the lithium-ion anode is present opposite to the cathode, it has a negative charge. Hence, it undergoes an oxidation reaction during the charging and discharging of the battery.
What Is Lithium Battery Anode Materials?
Like cathode materials, Anode materials can be of different types and serve different purposes. These materials are essential for storing and releasing energy during the charging and discharging of the batteries. So here are some commonly used Lithium-Ion anode materials;
- Lithium Alloyed Metals: these alloy metals have high stability and are safe to use. Hence, they are commonly used as anodes. One commercial application of it is lithium titanate (Li4Ti5O12).
- Carbon-Based Materials: The most commonly used material for anode is Graphite. It has a layered structure and smoothly allows lithium ions to intercalate during the charging. Moreover, its capacity for intercalation is limited, which affects its performance.
- Non-Graphitic Carbon Materials: Materials like amorphous and hard carbon are included. So, they have lesser initial capacities than graphite. However, they offer higher reversible capacities and longer life cycle stability.
- Novel Graphite Anodes: Researchers are searching for modified graphite forms, like kish graphite. It has high electrochemical properties. The purpose of the modified forms is to improve the performance and stability of graphite-based anodes.
- Silicon-Based Anodes: Silicon has a high theoretical capacity for lithium storage. So this makes it a suitable option for anode materials. However, as silicon goes through a large volume during the cycling process, the chances of electrode degradation are higher. Nanomaterials are also investigated to overcome this challenge because of their smaller volume.
So, the overall choice of materials for the lithium-ion battery anode and cathode is important to optimize the battery’s performance.
Part 6. FAQs
-
Can the cathode and anode of battery roles be reversed in a battery?
Yes, their role can be reversed in certain batteries during charging. For example, lithium-ion flows from the cathode to the anode during the charging. It is reversed while discharging the battery.
-
What is the lithium-ion battery working?
During the discharging of the lithium battery, lithium ions are released from the anode side and flow towards the cathode. So, it is the opposite when charging lithium-ion batteries. -
What are the major disadvantages of lithium-ion batteries?
Although the lithium battery serves the best cause, its drawbacks include being comparatively expensive, having more risk of bursting, being quite sensitive to high temperatures, and having a shorter life span. -
What is the expected lifespan of lithium batteries?
After proper care and adequate maintenance, a lithium-ion battery can last up to five years or 3000 charging cycles.
Related Tags:
More Articles

What You Need to Know About AA 3.6V Lithium Battery
Learn all about AA 3.6V lithium batteries—voltage, size, capacity, uses, and the best replacements. Discover why they’re powerful, and highly reliable.
What Are Lithium Salts and Why They Matter in Battery Electrolytes
Lithium salts in electrolytes are key to battery performance, powering everything from phones to EVs and shaping the future of clean energy.
Lithium AAA Battery Guide: Power, Performance & Chargers
Explore lithium AAA batteries—voltage, capacity, weight, top brands, and more. Learn how to choose the best battery for your device and why it really matters.
How to Calculate Watts, Volts, and Amps (With Simple Formulas and Examples)
Learn how to calculate watts, volts, and amps for lithium batteries with simple formulas and examples, ideal for EVs, solar, and energy systems.
Comprehensive Analysis of U.S. Tariffs on Chinese Lithium Batteries
U.S. tariffs on Chinese lithium batteries in 2025 impact costs, supply chains, and EV, energy storage, and electronics industries globally.




