Lithium-containing batteries, such as rechargeable batteries for mobile phones, computers, and electric vehicles, are widely used in our lives. At the same time, research on lithium-containing batteries is a hot spot in today’s scientific research and innovation. Therefore, we often hear concepts such as “lithium battery,” “lithium-ion battery,” and “lithium metal battery.” Are they the same thing?
Lithium metal batteries and lithium-ion batteries are both types of lithium batteries. So what is the difference between li-metal batteries and lithium-ion batteries? The following will tell you the difference between them in detail.
Part 1. Learn lithium-ion battery
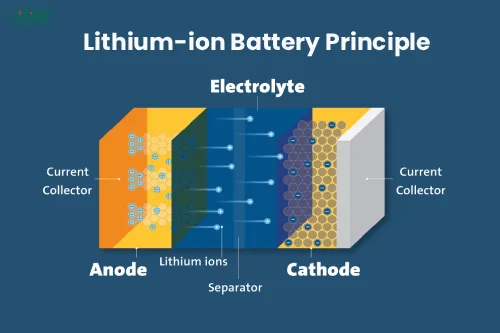
A lithium-ion battery is a secondary battery (rechargeable battery). It primarily relies on lithium ions moving between the positive and negative electrodes. During the charge and discharge process, Li+ intercalates and deintercalates back and forth between the two electrodes. When a lithium-ion battery is charged, Li+ is deintercalated from the positive electrode and embedded in the negative electrode through the electrolyte. The negative electrode is in a lithium-rich state. The opposite is true during discharge.
1 Lithium-ion battery composition
A. Positive electrode
The active material is generally lithium manganate, lithium cobalt oxide, and lithium nickel cobalt manganate material. Electric bicycles generally use lithium nickel cobalt manganate (commonly known as ternary) or ternary + a small amount of lithium manganate. Pure lithium manganate and lithium iron phosphate are gradually fading due to their large size, poor performance, or high cost. The conductive electrode fluid uses electrolytic aluminum foil with a thickness of 10-20 microns.
B. Diaphragm
A specially formed polymer film with a microporous structure. It allows lithium ions to pass through freely, but electrons cannot.
C. Negative electrode
The active material is graphite or has a graphite structure. The conductive current collector uses electrolytic copper foil with a thickness of 7-15 microns.
D. Organic electrolyte
A carbonate-based solvent in which lithium hexafluorophosphate is dissolved. Lithium polymer batteries use gel electrolytes.
E. Battery case
Lithium batteries are divided into steel shells (square type is rarely used), aluminum shells, nickel-plated iron shells (used in cylindrical batteries), aluminum-plastic films (soft pack batteries), etc. The battery cap is also the positive and negative terminal of the battery.
2 Working principle of lithium-ion battery
Lithium-ion batteries use carbon materials as the negative electrode and lithium-containing compounds as the positive electrode. There is no lithium metal, only lithium ions. This is a lithium-ion battery.
Lithium-ion batteries are the general term for using lithium-ion intercalation compounds as positive electrode materials. Lithium-ion batteries’ charging and discharging process is the intercalation and deintercalation process of lithium ions. During the intercalation and deintercalation process of lithium ions, it is also accompanied by the embedding and deintercalation of electrons equivalent to lithium ions (it is customary to express the positive electrode as insertion or deintercalation and the negative electrode as insertion or deintercalation). During the charging and discharging process, lithium ions are inserted/de-intercalated and inserted/de-inserted between the positive and negative electrodes, which is vividly called a “rocking chair battery.”
When a battery is charged, lithium ions are generated on the battery’s positive electrode. The generated lithium ions move to the negative electrode through the electrolyte. The carbon used as the negative electrode has a layered structure with many micropores. The lithium ions that reach the negative electrode are embedded in the micropores of the carbon layer. The more lithium ions are embedded, the higher the charging capacity.
Similarly, when the battery is discharged (that is, the process in which we use the battery), the lithium ions embedded in the carbon layer of the negative electrode are released and moved back to the positive electrode. The higher the discharge capacity, the more lithium ions returned to the positive electrode.
Generally, the charging current of a lithium battery is set between 0.2C and 1C. The larger the current, the faster the charging. At the same time, the battery generates more heat. Moreover, charging with a current that is too large will not fill the capacity because the electrochemical reaction inside the battery takes time. Just like pouring beer, pouring it too fast will produce foam and make it unsatisfactory.
3 Advantages of lithium-ion batteries
A. High voltage
The working voltage of a single lithium-ion battery cell is as high as 3.7-3.8V (the voltage of a lithium iron phosphate battery is 3.2V), three times that of Ni-Cd and Ni-MH batteries.
B. Specific capacity
The actual energy that the specific capacity of lithium-ion batteries can achieve is about 555Wh/kg. The material can achieve a capacity of more than 150mAh/g (3-4 times that of Ni-Cd, 2-3 times that of Ni-MH). It is close to about 88% of its theoretical value.
C. Long cycle life
Generally, it can reach more than 500 or even more than 1,000 times. Lithium iron phosphate batteries can reach more than 2000 times. For electrical appliances with low current discharge, the battery’s service life will double the electrical appliance’s competitiveness.
D. Safety
No environmental pollution, no memory effect. Lithium batteries, the predecessor of Li-ion, are prone to short-circuiting due to the tendency of metal lithium to form dendrites, which reduces their application fields. Moreover, Li-ion does not contain cadmium, lead, mercury, and other elements that pollute the environment. A major drawback of Ni-Cd batteries with some processes (such as a sintered type) is the “memory effect,” which seriously restricts the use of batteries. Still, Li-ion does not have this problem at all.
E. Small self-discharge
The self-discharge rate of fully charged Li-ion after one month of storage at room temperature is about 2%. Much lower than 25-30% of Ni-Cd and 30-35% of Ni-MH.
F. Fast charging
The capacity can reach over 80% of the nominal capacity after 30 minutes of 1C charging. Lithium iron phosphate batteries can be charged 90% of their nominal capacity in 10 minutes.
G. Working temperature
The operating temperature of lithium-ion batteries is -25~45°C. With improvements in the electrolyte and cathode, it is expected to be broadened to -40~70°C.
4 Disadvantages of lithium-ion batteries
A. Aging
Unlike other rechargeable batteries, lithium-ion batteries lose capacity slowly. This is related to the number of times the lithium-ion battery is used and also related to temperature. This decline phenomenon can be expressed by a decrease in capacity or an increase in internal resistance.
B. Recovery rate
About 1% of new products leaving the factory must be recycled for various reasons.
C. Intolerant of overcharging
When overcharging, excessive embedded lithium ions will be permanently fixed in the crystal lattice and cannot be released again, leading to short battery life.
D. Intolerant of over-discharge
During over-discharge, too many lithium ions are deintercalated from the electrode, which can cause the crystal lattice to collapse, thus shortening the lifespan.
Part 2. What is the lithium metal battery?
Lithium metal battery (LMB) is a battery that uses metallic lithium as the negative electrode (Anode). The matching positive electrode material can be oxygen, elemental sulfur, metal oxide, and other substances. Li-metal batteries work on the same principle as ordinary dry batteries. It uses metallic lithium as an electrode and generates electrical energy through the corrosion or oxidation of metallic lithium. Li-metal batteries are disposable and cannot be recharged after being used.
Because the electrodes of li-metal batteries use metallic lithium, their electrical energy is much greater than dry batteries made of other materials. This provides sufficient power for devices that require long-term power supply, such as portable devices such as cameras.
A lithium metal battery as a type of non-rechargeable (primary) battery that uses lithium in its pure metallic form as the anode. These batteries are known for their high energy density and long shelf life, making them ideal for applications where long-lasting power is required in a compact size.
1 Key Features of Li-Metal Batteries:
-
High Energy Density:
- Lithium metal batteries have a very high energy density compared to other battery types, such as alkaline or zinc batteries. This allows them to store more energy in a smaller, lighter package.
-
Non-Rechargeable:
- These are primary batteries, meaning they are designed for single-use and cannot be recharged. Once the battery is depleted, it must be replaced.
-
Voltage:
- The typical voltage of a lithium metal battery is around 3.0V, which is higher than most other types of single-use batteries (such as alkaline, which typically has 1.5V).
-
Long Shelf Life:
- Lithium metal batteries have an excellent shelf life, often up to 10–15 years when stored properly. This makes them ideal for devices that need long-term, reliable power without frequent replacements.
-
Low Self-Discharge Rate:
- These batteries have a low self-discharge rate, meaning they lose very little energy while not in use, which contributes to their long shelf life.
2 Common Applications:
- Medical devices: Pacemakers, hearing aids
- Consumer electronics: Watches, calculators, remote controls
- Military and aerospace: Due to their high energy density and reliability in extreme conditions
- Backup systems: Memory backup for computers and other electronics
- Safety and security devices: Smoke detectors, emergency beacons
3 Safety Considerations:
- Thermal Instability: Lithium metal is highly reactive, which can lead to thermal runaway or fire if the battery is damaged or improperly handled.
- Transport Restrictions: Due to safety concerns, there are restrictions on transporting lithium metal batteries, particularly by air.
Part 3. Lithium metal battery vs. lithium ion battery
This table provides a quick overview:
| Feature | Lithium-ion Battery | Lithium Metal Battery |
|---|---|---|
| Rechargeable | ✅ Yes | ❌ No |
| Energy Density | Moderate (~150–250 Wh/kg) | Very High (~300–500 Wh/kg) |
| Voltage | 3.6–3.8V | ~3.0V |
| Cycle Life | 500–2000 cycles | Single-use |
| Self-discharge | Low (~2% per month) | Very Low |
| Safety | Safer, but sensitive to high temp | Higher risk of thermal runaway |
| Applications | EVs, laptops, smartphones, energy storage | Watches, medical devices, backup systems |
| Cost | Moderate | Higher per Wh |
| Shelf Life | 2–5 years | 10–15 years |
The main difference between lithium metal batteries and lithium-ion batteries is that lithium metal batteries are disposable batteries. In contrast, lithium-ion batteries are rechargeable cycle batteries! The principle of lithium metal batteries is the same as that of ordinary dry batteries. It uses lithium metal as the electrode and generates electrical energy through the corrosion or oxidation of metallic lithium. It is useless after use and cannot be recharged.
Lithium-ion batteries generally use lithium cobalt oxide as the positive electrode, carbon as the negative electrode, and the electrolyte is filled in the middle to form a channel for free ions. A diaphragm separates the positive and negative electrodes to prevent short circuits. When charging, due to the action of the electric field, lithium ions swim out of the lithium cobalt oxide, pass through the pores in the separator in the electrolyte, and reach the negative electrode to react with carbon to form lithium carbide in the discharge process, in contrast, the lithium ions return to the positive electrode. It is the charging and discharging process of lithium-ion batteries.
From the electrochemical principles of the two, it can be found that the biggest difference between lithium-ion batteries and lithium metal batteries is that as long as they use Li+ ions as the “carrier” for ion migration between the positive and negative electrodes, they can be called lithium-ion batteries. Batteries that use lithium metal as an electrode are called lithium metal batteries.
However, li-metal batteries’ charge and discharge process is also accompanied by the migration of Li+ ions in the electrolyte. Therefore, strictly speaking, lithium metal batteries are a special type of lithium-ion batteries; that is, the concept of lithium-ion batteries includes lithium metal batteries. However, it is common in scientific papers to refer to “lithium-ion batteries,” generally non-lithium metal lithium-ion batteries. When lithium metal is used as the electrode, it is generally called a “lithium metal battery.”
Under such a definition, the distinction between the two is very clear. In lithium-ion batteries, lithium element only exists in the form of +1-valent Li+ ions, and no electron gain or loss occurs during the charge and discharge process. In lithium metal batteries, the lithium element will undergo corresponding valence changes during the charge and discharge process:
On some occasions, the concept of “lithium battery” has also appeared, including li-metal and lithium-ion batteries. It is a general term for batteries based on lithium elements.
Part 4. Li-ion vs li-polymer: what’s the difference?
Some people cannot distinguish between Li-ion batteries and Li-polymer batteries. The following table provides a simple comparison.
Li-ion batteries and Li-polymer (Li-Po) batteries are closely related:
| Feature | Li-ion | Li-polymer |
|---|---|---|
| Electrolyte | Liquid | Gel or solid polymer |
| Shape | Cylindrical, prismatic | Flexible pouch |
| Energy Density | High | Slightly lower |
| Safety | Moderate | Safer, less leakage |
| Applications | Smartphones, EVs | Drones, flexible electronics |
In short, Li-polymer is a variation of Li-ion, offering more design flexibility with slightly lower energy density.
Part 5. Lithium ion vs lithium metal: which is better?
- Rechargeable devices (EVs, smartphones, laptops): Lithium-ion batteries are preferred due to rechargeability, stability, and cost efficiency.
- Long-term primary power (pacemakers, remote sensors, watches): Lithium metal batteries are ideal due to high energy density and long shelf life.
- High-temperature or extreme environments: Li-ion batteries with specialized chemistry may work, but Li-metal batteries require careful handling due to safety risks.
In summary: Li-ion = rechargeable workhorse, Li-metal = high-density one-time power.
Part 6. FAQs
-
Are lithium metal batteries rechargeable?
No, lithium metal batteries are primary (non-rechargeable) batteries. Recharging them can cause the formation of lithium dendrites, leading to short circuits and potential safety hazards. -
What are the safety concerns with lithium metal batteries?
Lithium metal batteries can be susceptible to thermal runaway and explosion if damaged or overheated, as metallic lithium is highly reactive. -
Can lithium-ion batteries be used in high-temperature environments?
Lithium-ion batteries have a limited temperature range for safe operation, typically between -20°C to 60°C (-4°F to 140°F). High temperatures can degrade the battery and pose safety risks.
Related Tags:
More Articles

A Complete Guide to the Best Batteries for Flashlights
Compare the best batteries for flashlights, including AA, AAA, 18650, 21700, CR123A. See which battery offers the best brightness, runtime, and reliability.
How Long Do Rechargeable AA Batteries Last?
How long do rechargeable AA batteries last? Compare NiMH and lithium AA lifespan, recharge cycles, key factors, and performance vs alkaline batteries.
How Much Current Can a 9V Battery Really Supply?
Discover how many amps a 9V battery can supply, its actual current output, discharge rate, and capacity for alkaline, lithium, and rechargeable 9V batteries.
12V STD vs 12V AGM: Meaning, Differences, and Which Is Better
Understand what STD and AGM batteries mean, their key differences, and which 12V battery fits your needs best in 2026.
Battery Reconditioning Explained: A Comprehensive Guide
Learn what battery reconditioning is, how it works, how long it takes, and when reconditioning chargers are used for lead-acid and lithium-ion batteries.