- Part 1. What is a dry cell battery?
- Part 2. What is a wet cell battery?
- Part 3. Applications of dry cells
- Part 4. Applications of wet cells
- Part 5. Dry cell vs wet cell battery: 7 Key differences compared
- Part 6. 5 Pro maintenance tips for longer life
- Part 7. FAQs about wet cell battery and dry cell battery
Quick Answer: Dry cell batteries are compact, portable, and sealed, ideal for small electronics. Wet cell batteries contain liquid electrolytes, offer higher power, and are used in vehicles, boats, and backup systems. Understanding the differences between dry cell battery and wet cell battery helps you choose the right type for your needs.
Key Takeaways in 30 Seconds
- 🔋 Dry Cell Battery: No-spill paste design | 1.5V-9V | For portable electronics like flashlights and remotes
- ⚡ Wet Cell Battery: High power, liquid electrolyte | 6V-12V | For cars, boats, solar systems
- 🛑 Never use wet cells in consumer electronics
- 📈 About 72% of car batteries are wet cell type
Part 1. What is a dry cell battery?
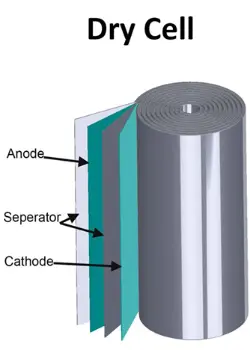
Dry Battery Composition and Structure
A dry cell battery is a compact, sealed battery that uses a paste electrolyte, making it safe for portable electronics. In contrast, a wet cell battery contains liquid electrolytes and is used in high-power applications such as automotive and solar systems.
Anode (Negative Electrode)
Typically made of zinc, the anode is the electrode where oxidation (loss of electrons) occurs during the battery’s discharge.
Cathode (Positive Electrode)
Usually composed of carbon or graphite mixed with manganese dioxide, the cathode is the electrode where reduction (gain of electrons) occurs during discharge.
Electrolyte
Dry cell batteries use a paste electrolyte instead of a liquid. This paste typically consists of a mixture of ammonium chloride and zinc chloride, serving as the medium for ion transfer between the anode and cathode.
Separator
A separator, often made of paper or similar material, is placed between the anode and cathode to prevent direct contact and short-circuiting while allowing ions to pass through.
Container
A sealed container made of zinc or steel encloses the entire assembly. This container protects the internal components and is the cathode terminal for making electrical connections.
Dry Battery Advantages and Disadvantages
Advantages
- Portability: Dry cell batteries are lightweight and compact, making them highly portable and suitable for a wide range of electronic devices, including flashlights, toys, and portable radios.
- No Leakage: Unlike wet cell batteries, which contain liquid electrolytes that can spill if the battery is damaged, dry cell batteries utilize immobilized electrolyte paste, reducing the risk of leakage and making them safer to handle.
- Durability: Dry cell batteries are generally more durable than wet cell batteries due to their sealed construction, which protects the internal components from damage and corrosion.
- Long Shelf Life: Dry cell batteries have a relatively long shelf life, retaining their charge for extended periods when not in use. The portability and long shelf life of dry cell batteries make them ideal for devices not used frequently or for emergency backup power.
Disadvantages
- Limited Capacity: Dry cell batteries typically have lower energy density and capacity than wet cell batteries. This characteristic means that dry cell batteries may last for a shorter duration in high-drain devices, necessitating more frequent replacements.
- Voltage Drop: As dry cell batteries discharge, their voltage gradually decreases, affecting the performance of devices that require a consistent power supply. This voltage drop may result in reduced performance or cause the device to shut down prematurely.
- Environmental Impact: Some dry cell batteries contain hazardous materials such as mercury or cadmium, which can harm the environment if not disposed of properly. Individuals or organizations should follow proper disposal methods for dry cell batteries to minimize environmental impact.
Part 2. What is a wet cell battery?
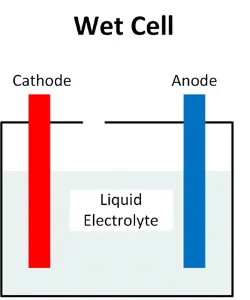
Wet Battery Composition and Structure
Wet cell batteries, also known as flooded cell batteries, contain a liquid electrolyte solution that facilitates the movement of ions between the anode and cathode. The composition and structure of a wet-cell battery include the following:
Anode (Negative Electrode)
The anode in a wet cell battery is typically made of lead (Pb). During discharge, lead undergoes oxidation, releasing electrons.
Cathode (Positive Electrode)
The cathode consists of lead dioxide (PbO2). Reduction occurs at the cathode during discharge, where lead dioxide gains electrons.
Electrolyte Solution
Unlike dry cell batteries, which use a paste electrolyte, wet cell batteries contain a liquid solution. This solution is typically a mixture of sulfuric acid (H2SO4) and water (H2O), facilitating the flow of ions between the anode and cathode.
Separator
Like dry cell batteries, a separator is used in wet cell batteries to prevent direct contact between the anode and cathode. This separator, often made of a porous material like rubber or plastic, allows ions to pass through while preventing short circuits.
Container
A durable plastic or rubber container houses the entire assembly. This container holds the electrolyte solution and provides structural support to the battery.
Wet Battery Advantages and Disadvantages
Advantages
- High Capacity: Wet cell batteries typically have higher energy density and capacity than dry cell batteries. Their ability to provide long-term, continuous power makes dry cell batteries suitable for applications such as in vehicles and backup power systems.
- Low Cost: Wet cell batteries are generally more cost-effective than dry ones. The materials used in wet cell batteries, such as lead and sulfuric acid, are readily available and inexpensive.
- Easy Maintenance: Wet cell batteries are relatively easy to maintain. Users can top dry cell batteries with distilled water to replenish electrolyte levels and extend their lifespan. This characteristic makes dry cell batteries suitable for automotive applications where regular maintenance is feasible.
- High Discharge Rates: Wet cell batteries can deliver high discharge rates, making them suitable for applications requiring sudden bursts of power, such as starting an engine in a vehicle or powering heavy machinery.
Disadvantages
- Risk of Spillage: One of the main disadvantages of wet cell batteries is the risk of electrolyte spillage. Since they contain liquid electrolytes, improper handling or damage to the battery can lead to leaks, which can be hazardous and corrosive.
- Weight and Size: Wet cell batteries tend to be larger and heavier than dry cell batteries due to their construction and the need for liquid electrolytes. This limitation can restrict their suitability for portable applications where size and weight are critical factors.
- Maintenance Requirements: Wet cell batteries are relatively easy to maintain, but they require regular maintenance to ensure optimal performance and lifespan. This maintenance process includes checking electrolyte levels, topping up with distilled water, and monitoring for signs of corrosion.
- Safety Concerns: Wet cell batteries contain sulfuric acid, a corrosive substance that can cause burns if it comes into contact with the skin or eyes. Handle and maintain wet cell batteries with proper safety precautions to prevent accidents and injuries.
Part 3. Applications of dry cells
Dry cell batteries are widely used in flashlights, remote controls, and portable electronics due to their compact size and reliability.
Due to their portability, reliability, and convenience, dry cell batteries are used in a wide range of electronic devices, including flashlights, remote controls, and portable electronics. Some typical applications include:
Flashlights
People widely use dry cells to power flashlights and torches because of their compact size and long shelf life. They provide reliable illumination for outdoor activities, emergencies, and everyday use.
Remote Controls
Many remote controls for televisions, air conditioners, and other electronic devices use dry cells for power. Their small size and lightweight make them ideal for handheld remote control units.
Portable Electronics
Many portable electronic devices rely on dry cells for power, including radios, MP3 players, and other electronic devices. Their portability and ease of use make them suitable for powering devices on the go.
Clocks and Watches
People commonly use dry cells in clocks, watches, and other timekeeping devices. They provide a reliable power source for maintaining accurate timekeeping without the need for frequent battery replacements.
Smoke Detectors
Many smoke and carbon monoxide detectors use dry cells for power. Their long shelf life and reliability make them well-suited for providing continuous power to these critical safety devices.
Calculators
People often use dry cells to power handheld calculators and other electronic devices in educational and professional settings. Their compact size and long-lasting power make them ideal for these applications.
Emergency Lighting
Dry cells, such as those used in exit signs and flashlights, are frequently employed in emergency lighting systems. They provide backup power during power outages or emergencies, ensuring continued illumination for safety and security purposes.
Portable Tools
Some portable tools, such as cordless drills, screwdrivers, and electronic measuring devices, are powered by dry cells. Their portability and convenience make them suitable for construction, maintenance, and DIY projects.
Part 4. Applications of wet cells
Wet cell batteries provide high-capacity, continuous power for vehicles, marine systems, and backup energy solutions.
Due to their high energy capacity and continuous power supply, wet cell batteries are used in automotive applications, marine systems, and telecommunications backup power. Some typical applications include:
Automotive Industry
Wet cell batteries are extensively used in vehicles, including cars, trucks, motorcycles, and boats, to provide the starting power for internal combustion engines. They also power auxiliary systems such as lights, radios, and air conditioning.
Marine Applications
Wet cell batteries are indispensable for marine vessels, providing power for engine start, navigation equipment, communication devices, and onboard appliances. Manufacturers design them to withstand the harsh marine environment and deliver reliable performance on watercraft.
Telecommunications
Wet cell batteries are employed in telecommunications infrastructure, including cellular towers, radio transmitters, and data centers, to provide backup power during grid outages or interruptions. They ensure uninterrupted communication services in critical situations.
Renewable Energy Systems
Wet cell batteries, such as those used in solar and wind power installations, are employed in renewable energy systems to store excess energy generated during peak production periods. They serve as energy storage solutions for off-grid and hybrid power systems.
Emergency Backup Power
Emergency backup power systems for critical facilities, such as hospitals, airports, and data centers, typically install wet cell batteries. They provide backup power during blackouts or emergencies, ensuring the continuous operation of essential services.
Industrial Equipment
Various industrial applications utilize wet cell batteries, including material handling equipment, uninterruptible power supplies (UPS), and backup generators. They provide reliable power for manufacturing processes, warehouse operations, and emergency power backup.
Electric Vehicles
Some electric vehicles, such as golf carts, forklifts, and scooters, use wet-cell batteries as their power source. These batteries provide the required energy density and power output for propulsion and auxiliary systems in electric vehicles.
Standby Power Systems
Wet cell batteries are used in standby power systems for residential, commercial, and industrial facilities to provide backup power during utility outages or fluctuations. They ensure continuity of operations and protect sensitive equipment from power disruptions.
Part 5. Dry cell vs wet cell battery: 7 Key differences compared
| Feature | Dry Cell Battery | Wet Cell Battery |
|---|---|---|
| Electrolyte Form | Paste/Gel (Non-spill) | Liquid Acid (Requires upright position) |
| Common Voltage | 1.5V (AA/AAA), 9V | 6V, 12V |
| Typical Cost | $0.5-$5 per unit | $50-$200+ |
| Lifespan (Unused) | 5-10 years | 2-5 years |
| Rechargeable | Mostly non-rechargeable | Usually rechargeable |
| Ideal For | Remote controls, flashlights | Car engines, solar systems |
| Maintenance | Zero maintenance | Monthly checkups |
| Electrolyte Form | Paste/Gel (Non-spill) – Typical Dry Cell Battery | Liquid Acid (Requires upright position) – Common Wet Cell Battery |
When to Choose Which? Decision Flowchart
Use this quick guide to find your perfect match:
🔘 Need power for portable devices? → Choose dry cell battery
🔘 Powering vehicle or marine equipment? → Choose wet cell battery
🔘 Outdoor use with vibration? → Gel Cell (Wet Cell subtype)
🔘 Emergency backup storage? → Deep Cycle Wet Cell Battery
Pro Tip: For RVs and boats, use a dual battery system – a dry cell for electronics and a wet cell for the engine.
Part 6. 5 Pro maintenance tips for longer life
For Dry Cells:
- Store in 15-25°C dry environment
- Remove when not in use for >1 month
For Wet Cells:
- Check electrolyte monthly (keep 1/4″ above plates) – Learn proper cleaning methods
- Clean terminals with baking soda solution
- Use smart charger for recharging
Part 7. FAQs about wet cell battery and dry cell battery
How to identify a wet cell battery vs. a dry cell battery?
Wet cells have removable caps and vent holes for liquid; dry cells are sealed and non-spill, making identification straightforward.
What type of battery lasts the longest?
Lithium dry cells can last up to 8-10 years in storage; well-maintained wet cells last 5-8 years in active use.
Can I use a dry cell battery in my car?
No. Cars need high current (200-800 CCA) that dry cells cannot provide. Always use wet cell or AGM batteries.
How often should I replace wet cell batteries?
Car batteries: 3-5 years, boat batteries: 4-6 years, solar storage: 5-8 years. Watch for slow starts or swelling cases.
Are wet cells more environmentally friendly?
Wet cells have a 98% lead recycling rate versus 30% for alkaline dry cells, but spilled acid can cause severe environmental harm.
Which is better for emergency kits?
Lithium dry cells like Energizer L91 are ideal. They last 20 years in storage and function in extreme temperatures.
Can wet cell batteries explode?
Yes, overcharging produces hydrogen gas. Always use vented battery boxes and proper ventilation in marine or RV setups.
Related Tags:
More Articles
What is the Difference Between Silver Zinc Battery vs. Lithium-ion Rechargeable?
Compare silver zinc and lithium-ion rechargeable batteries: energy density, cycle life, safety, cost, and uses in drones, medical devices, EVs, and electronics.
What are Watts and Watt Hours in Battery?
Understand watt vs watt-hour in batteries: key differences, how to calculate capacity, and why they matter. Includes free comparison table.
Best 10 Blood Pressure Monitor Battery Review: Finding the Most Reliable
Are you looking for a reliable Blood Pressure Monitor battery? Here is a complete guide with the top 10 best blood pressure monitor batteries.
Bluetooth Headphone Battery Guide: All You Need to Know
Maximize headphone battery life with expert tips! Learn how to charge, check, troubleshoot, and choose the best bluetooth headphone battery in 2025.
LiFePO4 Battery VS. Lithium-ion Polymer Battery: Which One Is Best?
Comprehensive comparison of LiFePO4 vs Lithium Ion Polymer batteries: energy density, safety, lifespan, cost. Find out which battery suits your needs in 2025.