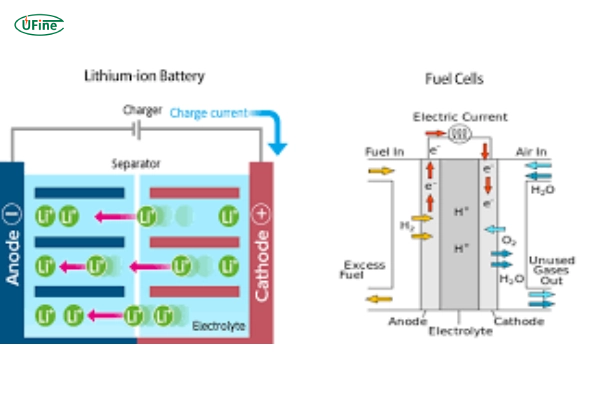
In the quest for sustainable energy solutions, fuel cells, and lithium-ion batteries have emerged as leading technologies. Both have unique strengths and weaknesses, making them suitable for different applications. This article compares these two technologies to help you understand which is better suited for specific needs.
Part 1. What is a fuel cell?
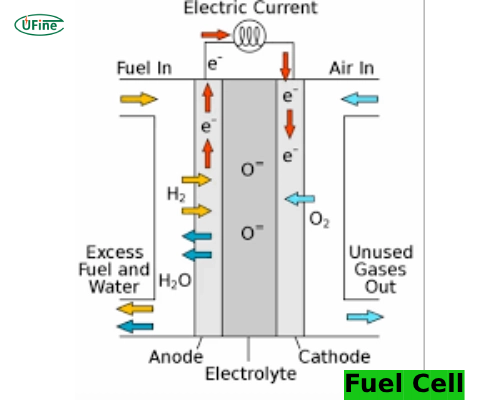
A fuel cell is an electrochemical device that converts the chemical energy of a fuel, typically hydrogen, directly into electricity. Unlike traditional combustion-based power generation, fuel cells generate electricity without burning fuel, making them more efficient and environmentally friendly.
How Does a Fuel Cell Work?
A fuel cell works by combining hydrogen and oxygen in an electrochemical reaction. Here’s a simplified breakdown of the process:
- Hydrogen Intake: Feed hydrogen gas into the fuel cell’s anode (positive side).
- Electrochemical Reaction: At the anode, hydrogen molecules into protons and electrons. The protons pass through an electrolyte membrane to the cathode (negative side). At the same time, the electrons travel through an external circuit, generating an electric current.
- Oxygen Intake: Feed oxygen from the air into the cathode.
- Water Formation: At the cathode, hydrogen protons, electrons, and oxygen combine to form water, expelling it as the only by-product.
This continuous process generates electricity as long as we supply hydrogen and oxygen.
Part 2. What is a lithium-ion battery?
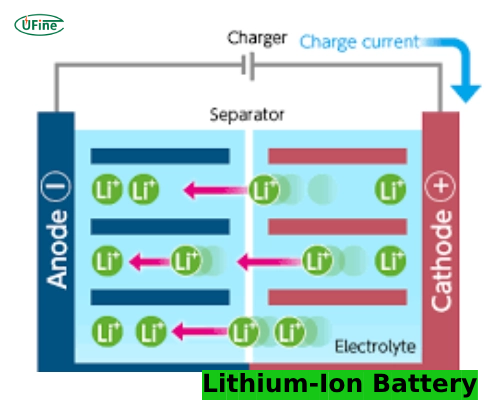
A lithium-ion battery is a type of rechargeable battery commonly used in consumer electronics, electric vehicles, and renewable energy storage systems. It operates on the principle of lithium-ion movement between the battery’s anode and cathode during charging and discharging cycles.
How Does a Lithium-Ion Battery Work?
Lithium-ion batteries consist of several key components:
- Anode (Negative Electrode): Typically made from graphite, batteries store lithium ions when they charge.
- Cathode (Positive Electrode): Made from lithium metal oxides, which release lithium ions during battery discharge.
- Electrolyte: A liquid or gel that allows lithium ions to move between the anode and cathode.
- Separator: A membrane that prevents direct contact between the anode and cathode, avoiding short circuits.
Lithium ions move from the cathode to the anode through the electrolyte during charging. When the battery is in use (discharging), ions return to the cathode, generating an electric current that powers devices.
Part 3. Pros and cons of fuel cells vs. Lithium-ion batteries
Advantages of Fuel Cells
- High Efficiency: Fuel cells can achieve up to 60% efficiencies, significantly higher than conventional combustion engines.
- Environmentally Friendly: The primary by-product of hydrogen fuel cells is water, making them a clean energy source with zero emissions.
- Scalability: Fuel cells can be scaled to power anything from small portable devices to large power plants.
- Quick Refueling: Hydrogen fuel cells can quickly refuel, similar to refilling a gasoline tank, making them ideal for transportation applications.
Advantages of Lithium-Ion Batteries
- High Energy Density: Lithium-ion batteries have a high energy density, which means they can store a large amount of energy in a compact size.
- Rechargeability: These batteries can recharge hundreds to thousands of times, making them cost-effective over their lifespan.
- Established Technology: Lithium-ion batteries are widely used and have a well-established supply chain and manufacturing process.
- Versatility: These batteries power various applications, from smartphones and laptops to electric vehicles and grid storage.
Disadvantages of Fuel Cells
- Hydrogen Production: Producing hydrogen, mainly green hydrogen (from renewable sources), is currently expensive and energy-intensive.
- Infrastructure: The infrastructure for hydrogen fueling stations needs to be developed more than electric charging stations.
- Storage and Transport: Hydrogen storage and transport pose significant challenges due to its low density and the need for high-pressure tanks.
Disadvantages of Lithium-Ion Batteries
- Limited Lifespan: Lithium-ion batteries degrade over time, losing capacity with each charge cycle.
- Environmental Impact: The mining of lithium and other materials used in batteries has significant environmental and ethical concerns.
- Safety Risks: Lithium-ion batteries can overheat and potentially catch fire if damaged or improperly handled.
- Disposal Issues: Proper disposal and recycling of lithium-ion batteries are critical to avoid environmental harm.
Part 4. Comparison between fuel cell vs lithium-ion battery
When comparing fuel cells and lithium-ion batteries, one must consider several factors: efficiency, environmental impact, cost, and application suitability. Below is a detailed comparison to help you understand the strengths and weaknesses of each technology.
1. Efficiency and Performance
Fuel Cells:
- Efficiency: Fuel cells can achieve up to 60% electrical efficiency, and combined heat and power (CHP) systems can reach 80-90% efficiency.
- Performance: Fuel cells provide consistent power output and perform well under various operating conditions.
Lithium-Ion Batteries:
- Efficiency: Lithium-ion batteries have a charge/discharge efficiency of around 85-95%.
- Performance: They offer high power density and are capable of rapid discharging and charging, which is crucial for applications requiring quick bursts of energy.
2. Environmental Impact
Fuel Cells:
- Emissions: The primary by-product of hydrogen fuel cells is water, resulting in zero emissions.
- Production: The environmental impact depends on the method of hydrogen production. Green hydrogen, produced using renewable energy, has minimal ecological impact.
Lithium-Ion Batteries:
- Emissions: While operating, lithium-ion batteries produce no emissions. However, their lifecycle emissions depend on the energy mix used for charging.
- Production: The extraction of lithium and other materials can have significant environmental and social impacts, including habitat destruction and pollution.
3. Cost Considerations
Fuel Cells:
- Initial Cost: High initial costs due to expensive materials (e.g., platinum catalysts) and manufacturing processes.
- Operating Cost: Hydrogen production and storage add to the operational costs, but technological advancements are expected to reduce these costs over time.
Lithium-Ion Batteries:
- Initial Cost: Relatively high but decreasing due to economies of scale and advancements in manufacturing.
- Operating Cost: Lower operating costs as they only require electricity for recharging. The user has asked not to rewrite the text.
4. Scalability and Infrastructure
Fuel Cells:
- Scalability: Highly scalable, suitable for applications ranging from portable electronics to large-scale power plants.
- Infrastructure: Limited hydrogen fueling infrastructure hinders widespread adoption, especially in transportation.
Lithium-Ion Batteries:
- Scalability: Widely used in various scales, from small electronic devices to large grid storage systems.
- Infrastructure: Well-established infrastructure for production, distribution, and charging, making them more accessible for consumers and industries.
5. Safety and Reliability
Fuel Cells:
- Safety: Hydrogen is highly flammable, requiring stringent safety measures for storage and handling.
- Reliability: Fuel cells are reliable with proper maintenance, providing consistent power output.
Lithium-Ion Batteries:
- Safety: Concerns over thermal runaway and potential fires, especially in large battery packs. Advances in battery management systems (BMS) help mitigate these risks.
- Reliability: Generally reliable but can degrade over time, reducing capacity and performance.
6. Applications and Use Cases
Fuel Cells:
- Transportation: Ideal for vehicles requiring extended range and quick refueling, such as buses, trucks, and trains.
- Stationary Power: Suitable for backup power systems, remote locations, and combined heat and power (CHP) applications.
Lithium-Ion Batteries:
- Consumer Electronics: Widely used in smartphones, laptops, and other portable devices.
- Electric Vehicles: Electric cars are the preferred choice due to their high energy density and established charging infrastructure.
- Grid Storage: Increasingly used for renewable energy storage and grid stabilization.
Part 5. FAQs
-
Are fuel cells better than lithium-ion batteries for electric vehicles?
Fuel cells and lithium-ion batteries offer different advantages for electric vehicles. Fuel cells are known for their extended range and quicker refueling than traditional batteries, making them suitable for larger vehicles like buses and trucks. However, lithium-ion batteries are currently more common in smaller electric cars due to their higher energy density and lower cost. -
How do fuel cells contribute to sustainable energy?
Fuel cells are a promising technology for sustainable energy production because they generate electricity through chemical reactions, typically involving hydrogen and oxygen. Unlike combustion engines, fuel cells produce electricity with minimal emissions—mainly water vapor and heat—making them a cleaner alternative for various applications, including stationary power generation and transportation. -
What are the main challenges facing the adoption of fuel cell technology?
Despite their advantages, the widespread adoption of fuel cells faces several challenges. Infrastructure for hydrogen refueling stations is limited, which restricts the deployment of hydrogen-powered vehicles. Additionally, the cost of producing and storing hydrogen remains relatively high compared to traditional fuels, posing economic barriers to broader adoption. -
How do lithium-ion batteries impact environmental sustainability?
Lithium-ion batteries are widely used in consumer electronics and electric vehicles due to their high energy density and rechargeable capabilities. However, the extraction of lithium and other raw materials for battery production can have environmental consequences, such as habitat disruption and water pollution. Recycling initiatives aim to mitigate these impacts and promote a circular economy for battery materials. -
What industries benefit most from fuel cell technology?
Fuel cells are finding applications across various industries, including automotive, telecommunications, and residential power generation. They are particularly advantageous in sectors requiring continuous power supply and where reducing emissions is a priority. For example, fuel cell-powered backup systems ensure reliable energy in telecommunications networks. In contrast, hydrogen fuel cell vehicles offer zero-emission transportation solutions.
Related Tags:
More Articles

How to Choose the Best Floor Scrubber Battery for Commercial Cleaning?
Selecting the ideal floor scrubber battery ensures a long runtime, rapid charging, and minimal maintenance for efficient commercial cleaning operations.
Battery for Blower vs Battery for Leaf Vacuum: Which One Should You Choose?
Battery for blower vs leaf vacuum—learn the key differences in power, fit, and runtime to choose the right battery for your outdoor tool needs.
How to Choose the Right Battery for Blower?
Choosing the right blower battery? Consider voltage, capacity, chemistry & usage. This guide helps match the best battery for peak performance.
How to Choose the Best Insulated Battery Box for Lithium Batteries?
Choosing the Best Insulated Battery Box for Lithium Batteries? Discover key factors such as size, material, and safety for optimal protection and performance.
7 Critical Elements on a Lithium Battery Shipping Label
What must be on a lithium battery shipping label? Learn 7 key elements to ensure safety, legal compliance, and correct handling across all transport modes.