Batteries power countless devices in our daily lives, but how does a battery work? At its core, a battery converts chemical energy into electrical energy to run gadgets like phones, cars, and more. This article explores the science behind batteries, their processes, and key factors influencing their performance. Whether you’re curious or a tech enthusiast, this comprehensive guide will clarify everything about battery operation.
Part 1. How does a battery work?
A battery works by converting chemical energy into electrical energy through a process called a redox reaction. When a battery discharges, electrons flow from the anode to the cathode through an external circuit, powering your device. During charging, this process is reversed, restoring the battery’s energy.
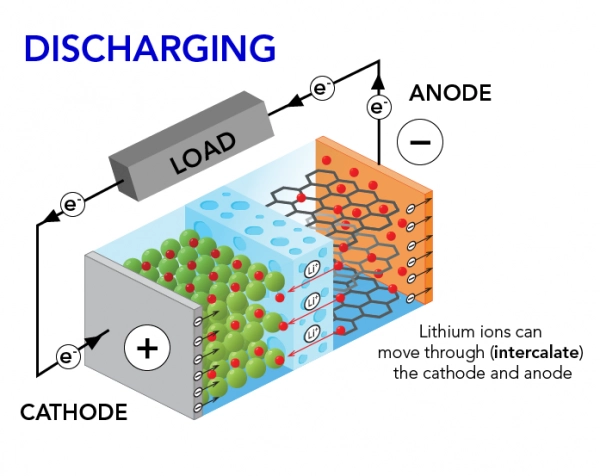
Battery Discharging Process
The discharging process in a battery occurs when stored chemical energy is released as electrical energy. This happens through a redox reaction where lithium ions move from the anode to the cathode, and electrons flow through the external circuit to power devices. This intricate process involves a series of chemical reactions within the battery that transform potential energy into electrical energy.
When a device is in use, the discharging process commences at the anode, a crucial component often composed of materials such as lithium, graphite, or metal oxides. During this phase, lithium ions are liberated from the anode, and electrons are set into motion. These electrons flow through the external circuit, creating an electric current that powers the connected device.
Simultaneously, lithium ions travel through the electrolyte to the cathode, where they embed themselves in the cathode material, commonly lithium cobalt oxide. This embedding process involves a reduction reaction, ensuring the completion of the electron flow within the battery.
The chemical reactions during discharging can be expressed as follows:
- Anode: LiCoO2→Li++CoO2+e−
- Cathode: LiC6+LiCoO2→Li2CO3+CoO2
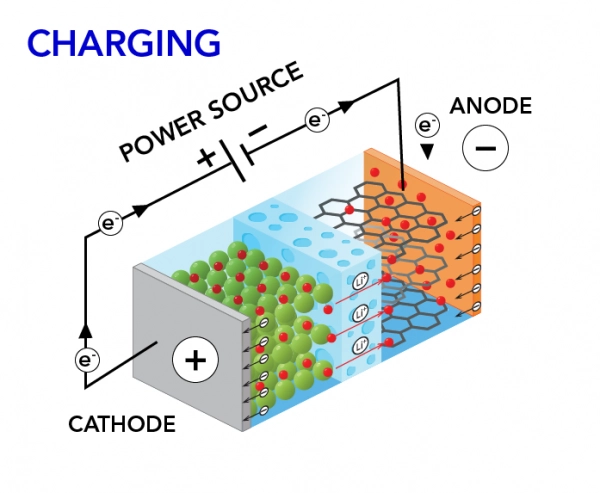
Battery Charging Process
The charging process is a critical phase where the battery replenishes its energy stores, ensuring it is ready for subsequent use. The charging process is initiated by connecting the battery to an external power source, such as an electrical outlet or a dedicated charging station.
During charging, the external power source applies a voltage to the battery, initiating a reversal of the chemical reactions that occurred during the discharging process. Lithium ions migrate from the cathode back to the anode, and electrons return to the anode through the external circuit. This cyclic process rejuvenates the battery, allowing it to store energy once again for future use.
The chemical reactions during the charging process can be summarized as follows:
- Anode: Li++CoO2+e− →LiCoO2
- Cathode: Li2CO3+CoO2→LiC6+LiCoO2
Part 2. The reaction inside a battery
Types of Batteries Explained
There are various types of batteries, each designed for specific applications:
- Primary Batteries: Non-rechargeable, such as alkaline batteries, commonly used in remote controls.
- Secondary Batteries: Rechargeable, like lithium-ion batteries found in phones and electric vehicles.
Artikel Terkait: What are Lithium Polymer Batteries?
2.1 Battery Chemical Reactions
At its core, a battery transforms chemical energy into electrical energy through a series of redox reactions. The two main types of batteries, namely rechargeable and non-rechargeable, exhibit distinct chemical processes.
Non-rechargeable battery
Such as an alkaline battery, zinc undergoes oxidation at the anode, releasing electrons into the external circuit. Simultaneously, at the cathode, manganese dioxide undergoes reduction by accepting these electrons, forming zinc oxide and water. This unidirectional flow of electrons sustains the battery’s power until the reactants are depleted, rendering the battery non-rechargeable.
Rechargeable batteries
Exemplified by lithium-ion batteries, engage in reversible redox reactions. During discharge, lithium ions move from the anode, typically composed of graphite, to the cathode, often containing lithium cobalt oxide. This movement of ions results in the release of electrical energy. During recharge, the process is reversed, emphasizing the dynamic nature of the chemical reactions within a rechargeable battery.
2.2 Battery Anode and Cathode
The elements in a battery’s performance are the anode and cathode, each playing a pivotal role in the battery’s operation. The anode, often composed of a metal or material that readily undergoes oxidation, serves as the site for electron release during the battery’s discharge phase. In contrast, the cathode, usually a material that easily undergoes reduction, welcomes these electrons during discharge.
For instance, in a common alkaline battery, zinc functions as the sacrificial anode, willingly parting with its electrons. Meanwhile, manganese dioxide, the cathode, eagerly accepts these electrons, facilitating the reduction reaction. In rechargeable batteries, the roles are interchangeable during the charge and discharge cycles, underscoring the dynamic nature of the anode-cathode interplay.
2.3 Battery Electrolyte
Central to the smooth operation of a battery is the electrolyte,which is a conductor of ions essential for completing the electrical circuit within the cell. Typically a liquid or gel, the electrolyte facilitates the flow of ions between the anode and cathode, enabling the redox reactions that generate electrical energy.
In lithium-ion batteries, for instance, a lithium salt dissolved in a solvent serves as the electrolyte. During discharge, lithium ions move through the electrolyte from the anode to the cathode, maintaining the overall charge balance within the system. The electrolyte’s composition directly influences the battery’s performance characteristics, including its conductivity, safety, and ability to operate over a range of temperatures
Part 3. Factors affecting battery performance
1. Temperature
Extreme temperatures can impact battery performance. High temperatures can accelerate chemical reactions, leading to faster degradation, while low temperatures can reduce battery capacity temporarily.
2. Discharge Rate
The rate at which a battery is discharged affects its performance. Higher discharge rates can lead to a decrease in overall capacity and efficiency.
3. Cycling
Cycling refers to the process of discharging and recharging a battery. The number of cycles a battery undergoes can affect its lifespan and capacity retention.
4. Storage Conditions
Proper storage conditions, including temperature and charge level, can significantly impact a battery’s shelf life. Storing batteries in a cool, dry place at a partial charge is generally recommended for long-term storage.
Part 4. Conclusion
Understanding the battery work is crucial not only for comprehending the science behind battery operation but also for optimizing battery usage, enhancing longevity, and contributing to the ongoing advancements in battery technology. These processes underscore the dynamic nature of batteries, making them indispensable components in our modern electronic landscape.
Key Takeaways
- Batteries work through a chemical reaction that converts stored energy into electrical energy.
- The discharging and charging processes involve the movement of ions and electrons between the anode and cathode.
- Temperature, discharge rate, and storage conditions play significant roles in battery performance and longevity.
Part 5. FAQs
-
How do batteries start?
The battery performs a chemical reaction that releases energy. The energy is released in the form of electricity, and the reaction can occur when the battery is connected to the circuit. -
Why do batteries go flat?
Batteries discharge and “go flat” because the chemical reactions that produce electrical energy within them eventually reach a point where the materials inside the battery are no longer able to generate a voltage. -
Do lithium batteries have memory?
No, modern lithium-ion batteries, which are commonly used in electronic devices, do not exhibit a memory effect. Memory effect was a concern with older rechargeable battery technologies, but it’s not a significant issue with lithium-ion batteries. -
Is it a battery AC or DC Current?
Batteries provide direct current (DC). DC flows in one direction, from the positive to the negative terminal of the battery. This is in contrast to alternating current (AC), which periodically changes direction. -
What is the liquid inside a battery?
The liquid inside a battery is called the electrolyte. In lead-acid batteries, commonly used in cars, the electrolyte is a solution of sulfuric acid and water. In lithium-ion batteries, the electrolyte is typically a lithium salt dissolved in a solvent.
Related Tags:
More Articles
Paper Battery vs. Flexible Battery: What’s the Difference and Which Is Better?
Paper vs. flexible batteries: learn the key differences, benefits, and which power source fits best for wearables, sensors, and smart tech.
What to Know Before Buying a Tiny LiPo Battery for Your Project
Tiny LiPo batteries are powerful and compact. Learn how to choose the right one for your project with specs, safety, and charging tips.
Bloated LiPo Battery: Will It Explode?
Will a bloated LiPo battery explode? Discover the causes, risks, safety steps, and expert tips to avoid disaster and protect your gear. Must-read safety guide!
12V 100Ah Lithium Ion Battery Price: Full Guide
Learn about 12V 100Ah lithium-ion battery price, from cost ranges to best brands, hidden fees, and how to get the best deal. A must-read for smart buyers!
Resistance and Conductivity: What It Means for Your Lithium Batteries
Resistance and conductivity impact lithium battery performance, lifespan, and safety—learn how they work and why they matter.