- Part 1. What is lithium?
- Part 2. Why is lithium important?
- Part 3. How is lithium mined?
- Part 4. Lithium mines for batteries
- Part 5. Emerging technologies in lithium mining
- Part 6. How does direct lithium extraction from saltwater work?
- Part 7. What are the benefits of geothermal brine extraction for lithium?
- Part 8. How does the ion exchange bead method improve lithium extraction efficiency?
- Part 9. Environmental impacts of lithium mining
- Part 10. FAQs
Lithium is critical in today’s technology, especially for electric vehicles and portable electronic batteries. As the demand for lithium rises, understanding how miners extract it becomes essential. This article will delve into the various methods of lithium extraction, the benefits of new technologies, and the environmental impacts associated with lithium mining.
Part 1. What is lithium?
Lithium is a soft, silvery-white metal that belongs to the alkali metal group on the periodic table. It is known for its high reactivity and flammability. Due to its unique properties, lithium is an excellent conductor of electricity and has a high energy density, making it ideal for use in rechargeable batteries. Lithium primarily occurs in two forms: brine and hard rock deposits.
Where Do Lithium Batteries Come From?
Part 2. Why is lithium important?
Lithium plays a vital role in several industries:
- Energy Storage: Lithium-ion batteries are essential for renewable energy storage solutions and electric vehicles.
- Lightweight: As one of the lightest metals, lithium helps reduce the overall weight of battery systems.
- High Energy Density: Lithium batteries can store a significant amount of energy relative to their weight, which enhances battery performance and longevity.
Part 3. How is lithium mined?
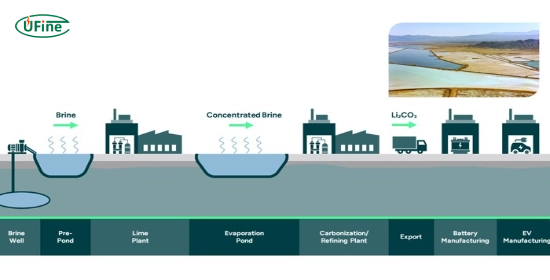
Lithium extraction occurs through two primary methods: brine extraction and hard rock mining. Each method has distinct processes and implications for the environment.
Brine Extraction
Brine extraction accounts for about 70% of global lithium production. This method involves extracting lithium from underground saltwater brines in salt flats or salars.
- Pumping: Workers pump naturally salty water from underground aquifers to the surface.
- Evaporation: They spread the brine across large evaporation ponds, allowing sunlight to evaporate the water over several months and concentrate the lithium.
- Precipitation: Technicians add chemicals to remove unwanted elements from the concentrated brine.
- Recovery: The resulting lithium-rich solution is processed to extract lithium carbonate or hydroxide.
This method is favored for its lower environmental impact than hard rock mining but raises concerns about water usage and ecosystem disruption.
Hard Rock Mining
Hard rock mining involves extracting lithium from mineral ores like spodumene, lepidolite, and petalite. This method is more labor-intensive and environmentally impactful than brine extraction.
- Crushing and Grinding: The ore is crushed into a fine powder to increase surface area for chemical reactions.
- Leaching: The powdered ore is treated with sulfuric acid or hydrochloric acid to dissolve lithium.
- Filtering and Concentrating: The leached solution is filtered to remove solid particles before concentrating it to remove excess water.
- Precipitation: Technicians add chemicals to precipitate lithium salts from the solution.
- Drying: The final product is filtered and dried to produce pure lithium compounds.
This method provides a higher yield of lithium but comes with significant environmental costs, including habitat destruction and high energy consumption.
Part 4. Lithium mines for batteries
Lithium mines are crucial for supplying the growing demand for batteries in electric vehicles (EVs) and other technologies. As countries push for greener alternatives, the need for reliable sources of lithium has become more pressing. Major lithium-producing regions include:
- Australia: Miners in Australia focus on hard rock mining, producing significant amounts of spodumene concentrate for battery production.
- Chile and Argentina: These countries extract brine from their vast salt flats (cellars), which are famous worldwide.
The global shift towards electric vehicles has led to increased investments in lithium mining operations worldwide, aiming to secure a stable supply chain for battery manufacturers.
Part 5. Emerging technologies in lithium mining
Researchers are developing innovative technologies to enhance lithium extraction efficiency while reducing environmental impacts.
- Direct Lithium Extraction (DLE): This method bypasses evaporation ponds using ion-exchange materials or membranes to extract lithium directly from brine sources.
- Geothermal Brine Extraction: This method utilizes geothermal energy to extract lithium from hot brines while generating electricity, thus providing a dual benefit.
- Redox-Couple Electrodialysis (RCE): A new technique that uses electricity to move lithium ions through membranes, significantly improving efficiency and reducing energy consumption compared to traditional methods.
Part 6. How does direct lithium extraction from saltwater work?
Direct Lithium Extraction (DLE) is an innovative method allowing more efficient lithium extraction from saltwater sources than traditional evaporation methods. Here’s how it works:
- Brine Pumping: Workers pump saltwater brine from underground sources into a processing facility.
- Ion Exchange Process: Specialized materials or beads absorb lithium ions selectively from the brine while leaving other minerals behind.
- Stripping Lithium: After absorption, the beads are treated with water or acid to release the captured lithium ions into a concentrated solution.
- Final Processing: The concentrated solution undergoes further processing to produce battery-grade lithium carbonate or hydroxide.
This method significantly reduces the time needed for extraction compared to traditional evaporation ponds, which can take months.
Part 7. What are the benefits of geothermal brine extraction for lithium?
Geothermal brine extraction offers several advantages over conventional methods:
- Sustainability: Geothermal energy production uses heat from the Earth, making it a renewable resource that can reduce reliance on fossil fuels while simultaneously extracting valuable minerals like lithium.
- Lower Environmental Impact: This method minimizes land disturbance and water usage compared to traditional hard rock mining or extensive evaporation ponds.
- Cost Efficiency: By integrating lithium extraction with geothermal power generation, companies can lower operational costs and create additional revenue streams from electricity and lithium sales.
Part 8. How does the ion exchange bead method improve lithium extraction efficiency?
The ion exchange bead method enhances lithium extraction efficiency through several key features:
- Selective Absorption: Manufacturers design porous ion exchange beads to attract and hold lithium ions while letting other unwanted minerals pass through.
- Reduced Processing Time: This method can extract lithium much faster than traditional techniques, often completing processes that would take months in just hours or days.
- High Recovery Rates: By optimizing conditions within the bead structure, companies can achieve higher lithium recovery rates from low-concentration solutions previously deemed unviable for extraction.
Part 9. Environmental impacts of lithium mining
While necessary for modern technology, lithium mining poses significant environmental challenges:
- Water Usage: Brine extraction consumes vast amounts of freshwater, impacting local ecosystems and communities reliant on these water sources.
- Carbon Emissions: Mining processes can emit substantial CO2; one ton of mined lithium can generate nearly 15 tons of CO2 emissions due to energy-intensive processes.
- Habitat Destruction: Both brine extraction and hard rock mining can lead to significant land degradation and loss of biodiversity.
Part 10. FAQs
-
What Are the Main Methods of Lithium Extraction?
The primary methods of lithium extraction are brine extraction and hard rock mining. Brine extraction involves pumping salty water from underground sources, while complex rock mining extracts lithium from minerals like spodumene. -
How Long Does It Take To Extract Lithium Using Brine?
The brine extraction process can take several months as it relies on evaporation ponds where water must evaporate naturally under sunlight before concentrating the lithium. -
What Are the Environmental Concerns Associated With Lithium Mining?
Environmental concerns include excessive water use, habitat destruction, carbon emissions during mining processes, and potential pollution from chemical leaching. -
Can Lithium Be Recycled?
Yes! Researchers are developing recycling methods for lithium-ion batteries to recover valuable materials like lithium, cobalt, and nickel, reducing reliance on newly mined resources. -
Where Are Major Lithium Deposits Located?
The “Lithium Triangle,” which includes parts of Chile, Argentina, and Bolivia, contains significant deposits. Australia also holds substantial complex rock lithium resources.
Related Tags:
More Articles

Battery Load Test: A Comprehensive Guide
Step-by-step battery load test guide for car, solar & industrial use. Learn how to load test a battery, interpret voltage charts, and avoid common mistakes.
The Comprehensive Guide to Battery Balancing and Battery Balancer
Discover how battery balancers improve lithium battery performance, lifespan, and safety. Learn types, functions, and tips to choose the right balancer.
What Is the Best Voltage for a Chainsaw Battery?
Compare 12V-80V chainsaw batteries for light pruning, medium firewood, and professional cutting. See best battery chainsaw with runtime charts and safety tips.
Lithium VS. Alkaline Batteries: A Comprehensive Comparison
Lithium batteries last 3–7× longer than alkaline and perform better in cold weather. Compare lifespan, cost, safety, and best uses to choose the right battery.
Comparing Lithium-Sulfur and Lithium-Ion Batteries: Which is Right for You?
Compare lithium-sulfur (Li-S) and lithium-ion batteries on energy, lifespan, cost, safety, and applications. Best choice for drones, EVs, and electronics.