- Part 1. What is manganese dioxide?
- Part 2. The role of manganese dioxide in alkaline batteries
- Part 3. Annual consumption of manganese dioxide
- Part 4. Sources of manganese dioxide
- Part 5. Environmental impact of manganese dioxide mining
- Part 6. Alternatives to manganese dioxide
- Part 7. How does the consumption of manganese dioxide in alkaline batteries compare to other types of batteries?
- Part 8. FAQs
Have you ever wondered how much manganese dioxide is consumed annually in alkaline batteries? This essential component plays a crucial role in the performance of these batteries, which power many household devices and electronic gadgets. Understanding the consumption of manganese dioxide sheds light on battery technology and highlights its significance in the global market.
Part 1. What is manganese dioxide?
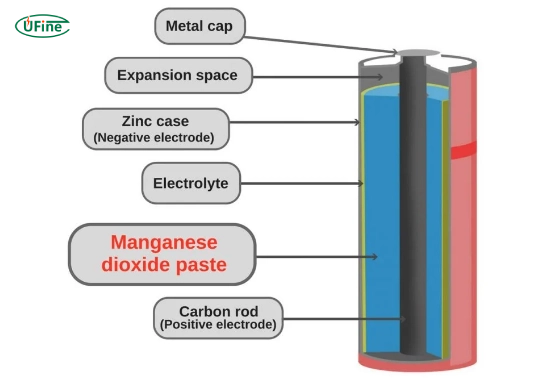
Manganese dioxide (MnO2) is a naturally occurring mineral that appears as a blackish-brown solid. Many industries widely use it, especially in battery applications. In alkaline batteries, manganese dioxide acts as a cathode material, which means it is involved in the chemical reactions that generate electricity.
Many manufacturers favor manganese dioxide for its excellent electrical properties and ability to facilitate electrochemical reactions. Its structure allows it to effectively store and release energy, making it an ideal choice for battery use. Without manganese dioxide, alkaline batteries would not function efficiently, leading to shorter lifespans and reduced energy output.
Part 2. The role of manganese dioxide in alkaline batteries
In alkaline batteries, manganese dioxide is the cathode, while zinc is the anode. The chemical reaction between these two components generates electricity, which powers our devices. The overall reaction can be simplified as follows:
- Zn+MnO2→ZnO+Mn2O3
During the discharge process, zinc oxidizes and releases electrons, while manganese dioxide reduces and accepts those electrons. This flow of electrons creates an electric current, allowing devices to harness power for various electronic functions.
The efficiency of this reaction depends heavily on the quality of manganese dioxide used. High-purity manganese dioxide ensures optimal performance and longevity of alkaline batteries. Manufacturers often seek out electrolytic manganese dioxide (EMD), produced through electrolysis, and offers superior purity compared to naturally sourced materials.
Part 3. Annual consumption of manganese dioxide
Research estimates that consumers purchase billions of alkaline batteries yearly, leading to a significant overall consumption of manganese dioxide. According to a report from the International Battery Association, around 50,000 tons of manganese dioxide are consumed annually in alkaline battery production worldwide. This staggering figure underscores the importance of this material in meeting global energy needs.
The demand for alkaline batteries has surged due to their widespread use in everyday items such as remote controls, toys, and flashlights. As technology advances and more devices require portable power sources, experts anticipate a further rise in manganese dioxide consumption.
Part 4. Sources of manganese dioxide
Manufacturers source manganese dioxide from natural mineral deposits and through industrial processing. Many manufacturers opt for electrolytic manganese dioxide (EMD) due to its higher purity, making it more suitable for battery applications. EMD is produced through electrolysis of manganese sulfate solutions, ensuring it meets the stringent quality requirements for adequate battery performance.
Natural sources include minerals like pyrolusite, primarily mined for its high manganese content. However, mining operations can have environmental impacts, increasing interest in recycling and sustainable sourcing methods.
Part 5. Environmental impact of manganese dioxide mining
The extraction of manganese dioxide can have ecological consequences, including habitat destruction and pollution. Mining operations can lead to soil degradation and water contamination if not managed properly. However, many mining companies adopt sustainable practices to minimize their ecological footprint.
Efforts include reducing water usage during extraction processes and implementing measures to prevent runoff from mining sites. Additionally, some companies are investing in research to improve recycling methods for used batteries, which could help reduce the demand for newly mined manganese dioxide.
Part 6. Alternatives to manganese dioxide
With technological advancements, researchers are exploring alternatives to manganese dioxide in alkaline batteries. Materials like lithium and sodium-based compounds show promise as potential substitutes due to their unique properties and advantages.
Lithium-ion batteries have gained popularity for their high energy density and longer lifespan compared to traditional alkaline batteries. However, they come with challenges, including higher costs and environmental concerns related to lithium extraction.
Sodium-based batteries are also being researched as a more sustainable alternative since sodium is abundant and less expensive than lithium or manganese. These alternatives may reshape the future of battery technology while reducing reliance on traditional materials like manganese dioxide.
Part 7. How does the consumption of manganese dioxide in alkaline batteries compare to other types of batteries?
When comparing the consumption of manganese dioxide in alkaline batteries to other types of batteries, such as lithium-ion or lead-acid batteries, notable differences emerge. Due to its effectiveness as a cathode material, alkaline batteries primarily rely on manganese dioxide as a key component.
In contrast, lithium-ion batteries utilize lithium cobalt oxide or lithium iron phosphate as cathodes, significantly reducing their dependence on manganese compounds. Lead-acid batteries rely on lead oxide instead of manganese compounds altogether.
While alkaline batteries consume around 50,000 tons of manganese dioxide annually, lithium-ion battery production does not utilize this material but instead focuses on lithium sources. This difference highlights how various battery technologies impact the demand for specific materials like manganese dioxide.
Part 8. FAQs
-
What is manganese dioxide used for in batteries?
Manganese dioxide acts as the cathode in alkaline batteries, generating electricity through a chemical reaction with zinc. -
How much manganese dioxide is consumed in alkaline batteries annually?
According to reports from the International Battery Association, approximately 50,000 tons of manganese dioxide are consumed annually worldwide in alkaline battery production. -
Are there alternatives to manganese dioxide in battery production?
Researchers are exploring materials such as lithium and sodium compounds as alternatives to manganese dioxide. -
What are the environmental impacts of manganese dioxide mining?
Mining for manganese dioxide can lead to habitat destruction and pollution; however, regulations are in place to minimize these impacts through sustainable practices. -
How does the demand for alkaline batteries affect manganese dioxide consumption?
The increasing demand for alkaline batteries directly correlates with the need for more manganese dioxide, especially with emerging technologies like electric vehicles.
Related Tags:
More Articles

What are Watts and Watt Hours in Battery?
Understand watt vs watt-hour in batteries, how to calculate battery watt hours, and what Wh means for car batteries, devices, and energy storage.
A Complete Guide to the Best Batteries for Flashlights
Compare the best batteries for flashlights, including AA, AAA, 18650, 21700, CR123A. See which battery offers the best brightness, runtime, and reliability.
How Long Do Rechargeable AA Batteries Last?
How long do rechargeable AA batteries last? Compare NiMH and lithium AA lifespan, recharge cycles, key factors, and performance vs alkaline batteries.
How Much Current Can a 9V Battery Really Supply?
Discover how many amps a 9V battery can supply, its actual current output, discharge rate, and capacity for alkaline, lithium, and rechargeable 9V batteries.
12V STD vs 12V AGM: Meaning, Differences, and Which Is Better
Understand what STD and AGM batteries mean, their key differences, and which 12V battery fits your needs best in 2026.