Batteries are essential in various applications, from consumer electronics to electric vehicles. Understanding how to manufacture different types of batteries is crucial for manufacturers aiming to innovate and improve battery technology. This guide provides a comprehensive overview of the materials, tools, and detailed steps involved in producing several types of batteries, with a focus on lithium-ion batteries.
Part 1. What is a battery?
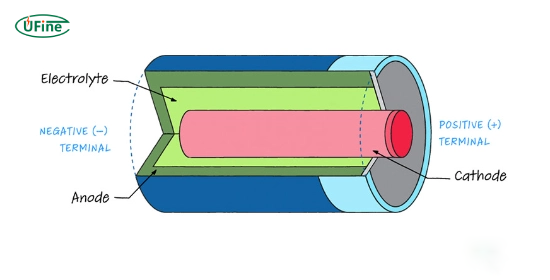
A battery is an electrochemical device that stores and converts chemical energy into electrical energy. It consists of one or more electrochemical cells, each containing:
- Anode: The negative electrode where oxidation occurs.
- Cathode: The positive electrode where reduction takes place.
- Electrolyte: A substance that allows ions to move between the anode and cathode.
When you connect a battery to a circuit, a chemical reaction occurs. This reaction generates an electric current that powers devices. You can classify batteries into primary (non-rechargeable) and secondary (rechargeable) types.
Part 2. Types of batteries
Batteries can be categorized based on their chemistry and design. Here are some common types:
- Lithium-Ion Batteries: Smartphones, laptops, and electric vehicles widely use these batteries due to their high energy density and rechargeability. They deliver a lot of power in a small size.
- Lead-acid batteries: Lead-acid batteries, known for their reliability and cost-effectiveness, have been around for over 150 years and are commonly used in cars and backup power systems.
- Nickel-metal hydride (NiMH) Batteries: NiMH batteries are often used in hybrid vehicles and rechargeable household products. They balance energy density and cost well.
- Sodium-Ion Batteries: Emerging as an alternative to lithium-ion batteries, sodium-ion batteries use sodium ions instead of lithium. People consider them more sustainable because sodium is more abundant than lithium.
Part 3. Materials used in battery manufacturing
The materials required for battery production vary by type but generally include:
- Lithium Compounds: Such as lithium carbonate or lithium hydroxide for lithium-ion batteries. These compounds are essential for the cathode.
- Graphite: Used for the anode due to its excellent conductivity and ability to intercalate lithium ions during charging.
- Metal Oxides: Manufacturers commonly use these in cathodes; examples include lithium cobalt oxide (LCO) and nickel manganese cobalt (NMC). These materials help determine the battery’s voltage and capacity.
- Electrolytes: Usually composed of lithium salts dissolved in organic solvents for lithium-ion batteries. The electrolyte facilitates ion movement between the electrodes.
- Binders and Conductive Additives: These include polyvinylidene fluoride (PVDF) for binding active materials together and carbon black to enhance conductivity.
Part 4. Manufacturing process of lithium-ion batteries
The battery production process for lithium-ion batteries involves several critical steps:
Step 1: Raw Material Extraction
- The first step is sourcing raw materials like lithium, cobalt, nickel, and graphite. These materials must be processed and refined before being used in battery production. Lithium is often extracted from brine pools or hard rock mining.
Step 2: Active Material Synthesis
- Chemical processes synthesize active materials for the anode and cathode. Natural or synthetic graphite is typically used for the anode, while metal oxides like lithium cobalt oxide or nickel manganese cobalt (NMC) serve as cathodes.
Step 3: Electrode Manufacturing
This process includes several sub-steps:
- Slurry Preparation: Mix active materials with binders and solvents to create a slurry and coat it onto metal foils.
- Coating: The slurry is coated onto metal foils—copper for anodes and aluminum for cathodes—using techniques such as slot-die coating or gravure printing.
- Drying: Excess solvent is removed through controlled drying processes to ensure optimal electrode performance.
Step 4: Calendering
- The coated electrodes are passed through rollers (calenders) to achieve the desired thickness and density. This step enhances the contact between particles, improving conductivity and overall battery performance.
Step 5: Slitting
- After drying, manufacturers cut electrode sheets into specific dimensions suitable for cell assembly. Precision in this step is crucial for ensuring uniformity across all cells produced.
Step 6: Cell Assembly
- The electrodes are stacked or wound together with a porous separator that prevents direct contact between the anode and cathode while allowing ion flow. The assembly process may vary depending on whether the battery is cylindrical, prismatic, or pouch-shaped.
Step 7: Formation
- After assembly, the cells undergo initial charging cycles known as formation cycling. This process helps form a solid electrolyte interphase (SEI) layer on the anode surface, critical for long-term battery performance.
Step 8: Aging
- Cells are then stored under controlled conditions to stabilize their performance before testing. This aging process allows any remaining reactions within the cell to complete, ensuring reliable operation when deployed.
Part 5. Manufacturing process of other battery types
Lead-Acid Batteries
Materials Needed:
- Lead dioxide (PbO2) for the positive plate
- Sponge lead (Pb) for the negative plate
- Dilute sulfuric acid as the electrolyte
- Separators made from porous material like fiberglass or polyethylene
Steps:
- Plate Preparation: Lead plates are formed into grids and coated with lead dioxide or sponge lead.
- Assembly: Plates are stacked with separators in between to prevent short circuits.
- Electrolyte Filling: Add dilute sulfuric acid to fill the cells.
- Sealing: Seal the battery to prevent leakage.
- Formation Charging: The battery undergoes charging to activate chemical reactions within the plates.
Nickel-Metal Hydride (NiMH) Batteries
Materials Needed:
- Nickel hydroxide (Ni(OH)2) for the positive electrode
- Hydrogen-absorbing alloy (often a rare earth metal mixture) for the negative electrode
- Potassium hydroxide (KOH) as the electrolyte
- Separators made from non-woven fabric or porous polymer
Steps:
- Electrode Preparation: Apply nickel hydroxide paste to nickel foam substrates.
- Assembly: Place electrodes with separators in between.
- Electrolyte Filling: Add a potassium hydroxide solution.
- Sealing: Seal the cell to maintain pressure.
- Formation Charging: Initial charging activates the electrodes by facilitating ion exchange.
Part 6. Quality control in battery manufacturing
Quality control is vital throughout the manufacturing process to ensure safety and performance:
- Testing Electrical Performance: Before approval, each cell’s voltage, capacity, internal resistance, and cycle life must meet specified standards.
- Safety Testing: Batteries undergo rigorous safety tests such as thermal stability tests, short-circuit tests, and overcharge tests to prevent failures that could lead to overheating or fires.
Part 7. Innovations in battery technology
The battery industry continues to evolve with new technologies aimed at improving performance:
- Solid-State Batteries: These batteries use solid electrolytes instead of liquid ones, which can enhance safety by reducing flammability risks while potentially increasing energy density significantly.
- Alternative Chemistries: Research into sodium-ion batteries aims to provide a more sustainable option by utilizing abundant sodium resources instead of scarce lithium materials.
- Battery Management Systems (BMS): Advanced BMS technology helps monitor battery health, optimize charging cycles, and extend overall lifespan through intelligent management strategies.
Part 8. FAQs
-
What raw materials are essential for making lithium-ion batteries?
Lithium compounds, graphite, metal oxides (like cobalt or nickel), electrolytes, binders, and conductive additives are crucial in producing lithium-ion batteries. -
How long does it take to manufacture a lithium-ion battery?
The entire manufacturing process, from raw material extraction through final assembly and testing, can take several days before the product is ready for distribution. -
What safety measures are taken during battery production?
Manufacturers implement strict quality control protocols at every production stage, including safety testing such as thermal runaway and short-circuit assessments. -
Can batteries be recycled?
Yes, you can recycle many components of batteries effectively. Recycling processes can recover metals like lithium, cobalt, and nickel, helping to reduce waste and environmental impact. -
What innovations are shaping the future of battery technology?
Solid-state batteries offer higher energy densities and improved safety than traditional lithium-ion batteries; alternative chemistries like sodium-ion aim at sustainability while enhancing performance metrics across applications.
Related Tags:
More Articles

How to Choose the Best Floor Scrubber Battery for Commercial Cleaning?
Selecting the ideal floor scrubber battery ensures a long runtime, rapid charging, and minimal maintenance for efficient commercial cleaning operations.
Battery for Blower vs Battery for Leaf Vacuum: Which One Should You Choose?
Battery for blower vs leaf vacuum—learn the key differences in power, fit, and runtime to choose the right battery for your outdoor tool needs.
How to Choose the Right Battery for Blower?
Choosing the right blower battery? Consider voltage, capacity, chemistry & usage. This guide helps match the best battery for peak performance.
How to Choose the Best Insulated Battery Box for Lithium Batteries?
Choosing the Best Insulated Battery Box for Lithium Batteries? Discover key factors such as size, material, and safety for optimal protection and performance.
7 Critical Elements on a Lithium Battery Shipping Label
What must be on a lithium battery shipping label? Learn 7 key elements to ensure safety, legal compliance, and correct handling across all transport modes.