We’ve all been there—you grab an old remote, flashlight, or toy, only to find crusty white residue or a suspicious wet leak around the battery compartment. That’s battery acid, and it’s more than just a nuisance—it can be dangerous if mishandled.
But what exactly is battery acid? Why does it leak? And most importantly, how can you protect yourself and your devices from its corrosive effects?
Part 1. What is battery acid?
Defining Battery Acid: It’s Not Always “Acid”
The term “battery acid” is a bit misleading because not all battery leaks are acidic. Depending on the battery type, the leaking substance can be:
-
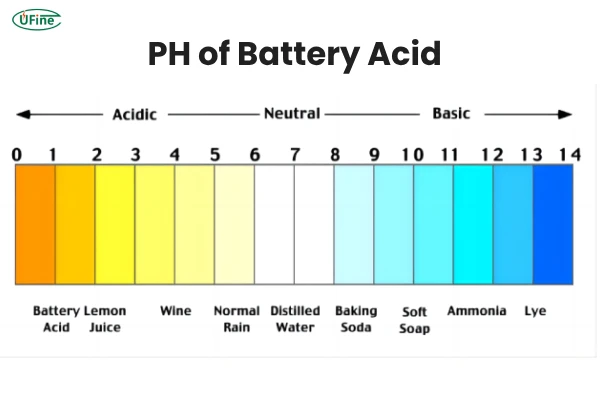
Alkaline (Basic): Found in AA, AAA, and other household batteries (potassium hydroxide, pH ~13-14)
-
Acidic: Found in lead-acid batteries like car batteries (sulfuric acid, pH ~0.8)
-
Other Electrolytes: Lithium batteries don’t contain “acid” but can leak harmful organic solvents
Battery acid is typically a clear, colorless liquid, but it can change over time.
- New battery acid – Transparent or slightly yellowish.
- Old or contaminated acid – Can turn brown, black, or cloudy due to chemical reactions and impurities.
- Leaked battery acid – Often leaves behind a white, crusty residue when dried.



What Does Battery Acid Look Like? Identifying Different Types
| Battery Type | Leak Appearance | Smell | Texture |
|---|---|---|---|
| Alkaline (AA, AAA) | White/greenish crust | Metallic, sharp | Powdery, crystalline |
| Lead-Acid (Car Batteries) | Oily, wet liquid | Rotten eggs (sulfur) | Slick, corrosive |
| Lithium (Li-ion/LiPo) | Dark oily residue | Sweet, chemical-like | Sticky, flammable |
Key Insight:
-
Alkaline leaks form crystalline deposits as potassium hydroxide reacts with air.
-
Lead-acid leaks are wet and oily because sulfuric acid is liquid at room temperature.
-
Lithium battery leaks are less common but more dangerous—they can catch fire if exposed to air.
If you’re looking for safe, leak-proof lithium battery solutions, Ufine Battery provides custom lithium-ion and LiPo battery options for various applications. Contact us today!
Part 2. Why do batteries leak acid?
Batteries don’t just leak randomly—there are specific chemical and physical reasons why battery acid escapes.
Primary Causes of Battery Leaks
A. Chemical Degradation Over Time
All batteries slowly break down due to:
-
Self-discharge: Even unused batteries lose charge, leading to internal pressure changes.
-
Electrolyte breakdown: The liquid inside batteries gradually decomposes, forming gas.
B. Physical Damage
-
Punctures or cracks (e.g., dropping a battery)
-
Swelling due to overcharging (common in cheap rechargeables)
C. Temperature Extremes
-
Heat accelerates chemical reactions, increasing internal pressure.
-
Freezing can crack battery casings, especially in lead-acid batteries.
D. Poor Manufacturing (Especially in Cheap Batteries)
Low-quality batteries often:
-
Use thin casings that rupture easily.
-
Lack proper pressure vents, leading to leaks.
Pro Tip:
If you want leak-proof reliability, consider Ufine Battery’s premium lithium-ion cells, which are sealed better and less prone to leakage than standard alkaline batteries.
Part 3. Which batteries are most likely to leak acid?
Not all batteries leak equally. Here’s a detailed breakdown of which types are most problematic:
A. Alkaline Batteries (AA, AAA, etc.)
-
Leak Risk: High (especially after expiration)
-
Why? Potassium hydroxide reacts with CO₂ in air, forming crystals that rupture the casing.
-
Common Culprits: Cheap off-brand batteries left in devices for years.
B. Lead-Acid Batteries (Cars, UPS Systems)
-
Leak Risk: Moderate (usually due to overcharging or damage)
-
Why? Sulfuric acid is highly corrosive and can eat through metal over time.
C. Lithium-Ion (Phones, Laptops, Power Tools)
-
Leak Risk: Low (but dangerous when it happens)
-
Why? They contain flammable electrolytes, not acid, but leaks can cause fires.
D. Lithium Polymer (LiPo) Batteries (Drones, RC Cars)
-
Leak Risk: Very low (if undamaged)
-
Why? Solid polymer electrolytes are more stable than liquid ones.
Takeaway:
If you want maximum safety, lithium-based batteries (especially from Ufine Battery) are the best choice for leak resistance.
Part 4. How dangerous is battery acid?
Battery acid isn’t just messy—it can cause serious harm if not handled properly.
A. Skin Contact
-
Immediate Effect: Chemical burns, redness, pain.
-
Long-Term Risk: Scarring or nerve damage (with severe exposure).
B. Eye Exposure
-
Immediate Effect: Extreme pain, blurred vision.
-
Long-Term Risk: Permanent corneal damage or blindness.
C. Inhalation (Lead-Acid Fumes)
-
Immediate Effect: Coughing, dizziness, lung irritation.
-
Long-Term Risk: Respiratory issues, lead poisoning.
D. Ingestion (Extremely Dangerous!)
-
Immediate Effect: Mouth/throat burns, vomiting.
-
Long-Term Risk: Esophageal damage, internal bleeding.
First Aid Summary:
✔ Skin: Rinse with water for 15+ minutes.
✔ Eyes: Flush with saline solution immediately.
✔ Inhalation: Move to fresh air, seek medical help.
✔ Ingestion: Do NOT induce vomiting—call poison control.
Part 5. Step-by-step: how to clean up battery acid safely
For Alkaline Battery Leaks (White Powder)
-
Put on gloves & goggles (safety first!).
-
Neutralize with vinegar (mild acid counteracts the alkaline leak).
-
Scrub gently with a toothbrush, then wipe with a damp cloth.
-
Dispose of residue in a sealed bag.
For Lead-Acid Battery Leaks (Wet Acid)
-
Sprinkle baking soda to neutralize the sulfuric acid.
-
Wait 5 minutes (it will bubble as it reacts).
-
Wipe up with paper towels, then rinse with water.
For Lithium Battery Leaks (Oily Residue)
⚠ Do NOT use water! (Lithium reacts violently with water.)
✔ Use sand or a Class D fire extinguisher if the battery is smoking.
The Ultimate Guide to Remove Battery Corrosion
Part 6. How to reduce the risk of battery acid leaks?
Prevention is always better than dealing with a dangerous acid spill. Here’s how you can minimize battery acid leaks:
✔ Store Batteries Properly – Keep them in a cool, dry place away from direct sunlight.
✔ Use the Right Charger – Overcharging leads to overheating and acid leaks.
✔ Inspect Regularly – Look for bulging, corrosion, or cracks in the battery casing.
✔ Dispose of Old Batteries – Don’t use expired or damaged batteries.
✔ Switch to Safer Alternatives – Consider using lithium batteries that do not leak acid.
For safe, high-quality lithium battery solutions, Ufine Battery offers custom lithium-ion and LiPo options. Contact us today for expert assistance!
Part 7. Proper disposal and recycling of batteries
Battery acid is not only dangerous to humans—it can also cause serious environmental damage if not disposed of correctly.
How to Dispose of Different Batteries
✔ Lead-Acid Batteries – Take them to auto shops or recycling centers. Many retailers accept old batteries.
✔ Lithium Batteries – Find a certified e-waste recycler to ensure proper disposal.
✔ Household Batteries (AA, AAA, etc.) – Check local regulations on whether they require special disposal.
✔ Damaged or Leaking Batteries – Place in a sealed plastic bag and take them to a hazardous waste facility.
By recycling, we help reduce pollution and conserve valuable materials.
Part 8. FAQs
Can battery acid corrode metal?
Yes, battery acid is highly corrosive to metal. It can eat through aluminum, steel, and other materials over time. If acid spills on metal surfaces, clean it immediately with baking soda and water to prevent permanent damage.
Is dried battery acid still dangerous?
Yes, dried battery acid can leave behind corrosive salts, which can reactivate when exposed to moisture. If you see a white or crystalline residue around a battery, do not touch it with bare hands. Wear gloves and clean it with a baking soda solution.
Can I mix water with battery acid?
Never add water directly to concentrated battery acid, as this can cause a violent reaction and generate extreme heat. Instead, when diluting acid (if necessary), always add acid to water slowly while stirring to prevent dangerous splashes.
Can battery acid damage the environment?
Yes, battery acid is highly toxic to soil and water. If spilled outdoors, it can contaminate groundwater and harm plants and wildlife. Always clean up spills properly and dispose of batteries through recycling programs.
Related Tags:
More Articles

How to Choose the Best Floor Scrubber Battery for Commercial Cleaning?
Selecting the ideal floor scrubber battery ensures a long runtime, rapid charging, and minimal maintenance for efficient commercial cleaning operations.
Battery for Blower vs Battery for Leaf Vacuum: Which One Should You Choose?
Battery for blower vs leaf vacuum—learn the key differences in power, fit, and runtime to choose the right battery for your outdoor tool needs.
How to Choose the Right Battery for Blower?
Choosing the right blower battery? Consider voltage, capacity, chemistry & usage. This guide helps match the best battery for peak performance.
How to Choose the Best Insulated Battery Box for Lithium Batteries?
Choosing the Best Insulated Battery Box for Lithium Batteries? Discover key factors such as size, material, and safety for optimal protection and performance.
7 Critical Elements on a Lithium Battery Shipping Label
What must be on a lithium battery shipping label? Learn 7 key elements to ensure safety, legal compliance, and correct handling across all transport modes.