- Part 1. What is a cathode?
- Part 2. Understanding electrical charges
- Part 3. The polarity of electrodes in different cells
- Part 4. How do cathodes and anodes differ in their roles within electrochemical cells?
- Part 5. Why is the cathode negative in some contexts?
- Part 6. The role of cathodes in everyday life
- Part 7. How do different materials affect cathodes?
- Part 8. Common misconceptions about cathodes
- Part 9. FAQs
Whether a positive or negative cathode is fundamental to understanding electrochemical cells and their functions. In physics and chemistry, electrodes are critical components that facilitate chemical reactions, and their polarities can vary based on the type of cell in which they operate. This article delves into cathodes’ characteristics, functions, and distinctions, clarifying their role in various electrochemical processes.
Part 1. What is a cathode?
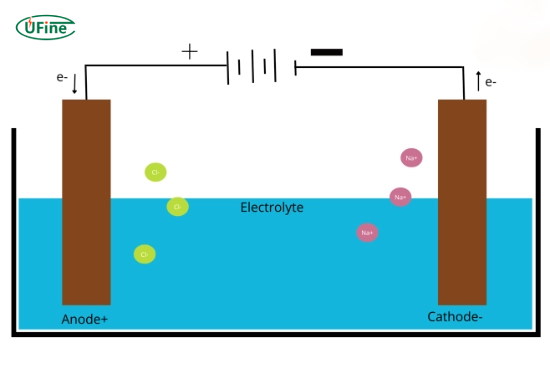
A cathode is defined as an electrode where reduction occurs. Simply put, it serves as a terminal through which current enters an electrochemical cell. This definition highlights its pivotal role in chemical reactions, contrasting with the anode, where oxidation occurs.
In a typical electrochemical setup, the cathode is the site for gaining electrons, essential for battery operation and electrolysis. The direction of electron flow is crucial here; electrons move from the anode to the cathode, making it vital to understand how these components interact within a circuit.
Part 2. Understanding electrical charges
To determine the polarity of a cathode, it’s essential to grasp electrical charges and their behavior in electrochemical processes. Electrons carry a negative charge and flow towards the positively charged anode.
- Electrons: Negatively charged particles that migrate from the anode to the cathode during reactions.
- Ions: Positively charged particles (cations) that travel toward the cathode as they seek to gain electrons.
This movement of electrons and ions is fundamental to maintaining electrical neutrality and facilitating chemical changes within the cell.
Part 3. The polarity of electrodes in different cells
Different types of electrochemical cells assign polarities differently to anodes and cathodes:
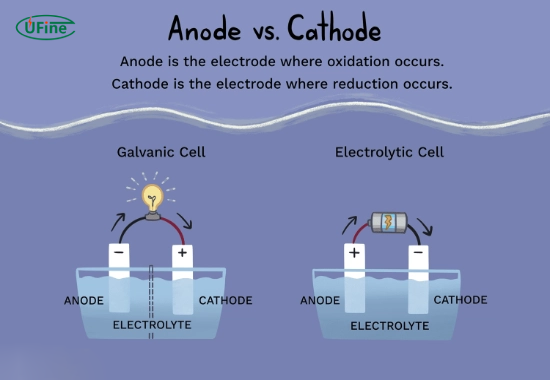
- Galvanic Cells: In these cells, which generate electrical energy from spontaneous chemical reactions, the anode is considered negative while the cathode is positive. This means that the cathode attracts cations from the electrolyte solution during discharge.
- Electrolytic Cells: These cells require an external power source to drive non-spontaneous reactions. While the cathode remains where reduction occurs, its polarity can be negative due to the external voltage applied.
Understanding these distinctions is key to grasping how various devices operate and how they can be utilized effectively.
Part 4. How do cathodes and anodes differ in their roles within electrochemical cells?
The roles of cathodes and anodes are distinct yet complementary within electrochemical cells:
- Cathodes: As previously mentioned, cathodes are where reduction occurs. They attract cations from the electrolyte and facilitate electron gain during chemical reactions. This process is crucial for battery energy storage and driving reactions in electrolytic cells.
- Anodes: Conversely, anodes are where oxidation takes place. They release electrons into the circuit while attracting anions from the electrolyte. The flow of electrons from the anode to the cathode creates a current that powers devices.
This interplay between cathodes and anodes is essential for understanding how batteries operate, and energy conversion occurs in various technologies.
Part 5. Why is the cathode negative in some contexts?
In certain situations—like in cathode ray tubes or during specific reactions—a cathode can initially appear as negative. This confusion arises because terms have subtle differences depending on industrial and scientific contexts.
For example, in a cathode ray tube (CRT), electrons are emitted from a heated filament (the cathode) and travel toward a positively charged anode. While the cathode is negatively charged due to electron emission, its role as a reduction site remains consistent with its definition.
Part 6. The role of cathodes in everyday life
Understanding the function of cathodes expands beyond science into everyday applications. For example:
- Batteries: Cathodes store and release energy during discharge and charge cycles. Lithium-ion batteries use specific materials for their cathodes that enhance efficiency and longevity.
- Electrolysis: In processes like water splitting, cathodes facilitate hydrogen production by reducing protons into hydrogen gas. This reaction has significant implications for renewable energy sources.
Recognizing these roles helps consumers better manage technologies that rely on electrochemical principles, such as electric vehicles or solar energy systems.
Part 7. How do different materials affect cathodes?
The material used for a cathode can significantly influence its performance in various applications:
- Carbon-Based Materials: Often used in batteries due to their conductivity and stability under various conditions.
- Metallic Cathodes: Nickel or platinum are employed in electrolytic cells because they efficiently facilitate electron transfer.
Choosing appropriate materials can enhance efficiency and longevity in fuel cells and batteries. Research into new materials continues to evolve rapidly, leading to advancements that improve performance across many applications.
Part 8. Common misconceptions about cathodes
Several misconceptions surround the concept of cathodes:
- Misconception 1: “Cathodes are always positive.” This is not true; while they are positive in galvanic cells, they can be negative in other contexts, like electrolytic cells.
- Misconception 2: “All electrodes function identically.” Each electrode has unique roles depending on its environment and application.
Clearing these misconceptions is vital for a deeper understanding of electrochemistry and its applications across various fields.
Part 9. FAQs
-
What makes a material suitable for use as a cathode?
A suitable material for a cathode should have high electrical conductivity, stability under operating conditions, and compatibility with other cell components to ensure efficient electron transfer. -
Is a cathode always negative?
A cathode can be negative in some contexts, like cathode ray tubes, but is generally considered positive in galvanic cells. -
What role does temperature play in a cathode’s performance?
Temperature affects reaction rates; higher temperatures can enhance conductivity but may also lead to degradation of materials over time. -
Can different types of batteries use other materials for their cathodes?
Yes, different types of batteries utilize various materials based on desired properties such as energy density, charge/discharge rates, and overall efficiency. -
How do advancements in technology impact cathode design?
Advancements lead to new materials that improve efficiency, reduce costs, and enhance safety features in devices like electric vehicles and portable electronics.
Related Tags:
More Articles

Battery Load Test: A Comprehensive Guide
Step-by-step battery load test guide for car, solar & industrial use. Learn how to load test a battery, interpret voltage charts, and avoid common mistakes.
The Comprehensive Guide to Battery Balancing and Battery Balancer
Discover how battery balancers improve lithium battery performance, lifespan, and safety. Learn types, functions, and tips to choose the right balancer.
What Is the Best Voltage for a Chainsaw Battery?
Compare 12V-80V chainsaw batteries for light pruning, medium firewood, and professional cutting. See best battery chainsaw with runtime charts and safety tips.
Lithium VS. Alkaline Batteries: A Comprehensive Comparison
Lithium batteries last 3–7× longer than alkaline and perform better in cold weather. Compare lifespan, cost, safety, and best uses to choose the right battery.
Comparing Lithium-Sulfur and Lithium-Ion Batteries: Which is Right for You?
Compare lithium-sulfur (Li-S) and lithium-ion batteries on energy, lifespan, cost, safety, and applications. Best choice for drones, EVs, and electronics.