- Part 1. What is lithium?
- Part 2. Is lithium a liquid, solid, or gas?
- Part 3. What are the physical states of lithium?
- Part 4. What makes lithium unique?
- Part 5. Why is lithium important in batteries?
- Part 6. Where is lithium found?
- Part 7. How is lithium processed?
- Part 8. Is lithium dangerous?
- Part 9. What are the other uses of lithium?
- Part 10. FAQs
Lithium plays a vital role in today’s world. Lithium is everywhere, from powering our phones and laptops to enabling the rise of electric vehicles. But have you ever wondered what physical state lithium exists in? Is it a liquid, solid, or gas? Understanding the fundamental properties of lithium is key to appreciating why it’s so important.
This article will explore lithium’s physical states, unique characteristics, and many applications. At the end, we’ll answer some frequently asked questions to clarify any confusion.
Part 1. What is lithium?
Lithium is a chemical element with the symbol Li and atomic number 3. It is part of the alkali metal group on the periodic table, a family of highly reactive metals. Lithium is the lightest metal and has a silvery-white appearance. Its low density and high reactivity make it a standout element.
Lithium is also unique because it is one of the few elements that naturally occur in trace amounts across the universe. People find it in rocks, brine pools, and even seawater. This lightweight metal is essential for energy storage, mental health treatments, and industrial applications.
In short, lithium is a tiny element with a significant impact. But to truly understand it, let’s look at how lithium behaves in different physical states.
Where Do Lithium Batteries Come From?
Part 2. Is lithium a liquid, solid, or gas?
The short answer? Lithium is a solid at room temperature and normal pressure. But like all elements, lithium can exist in other states—liquid or gas—under certain conditions.
Here’s a breakdown:
- Solid: Lithium is a soft, silvery metal at room temperature (around 20°C or 68°F). People can cut it with a knife, and it reacts quickly when exposed to air or water.
- Liquid: Lithium melts at 180.5°C (356.9°F), turning into a liquid. In this state, nuclear reactors often use it as a heat transfer agent.
- Gas: Lithium becomes a gas if heated to 1,342°C (2,448°F). Scientists rarely use it in its gaseous form but experimentally study this state. This ability to transform between states makes lithium a versatile element. However, people most commonly encounter it in its solid state.
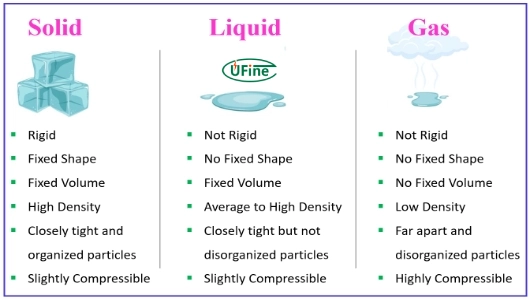
Part 3. What are the physical states of lithium?
Solid Lithium
Lithium is a solid under normal conditions, and it has some exciting properties in this state:
- Soft and lightweight: It’s so light that it can float on oil. Lithium is the least dense of all metals.
- Reactivity: Lithium reacts quickly with water, forming hydrogen gas and lithium hydroxide. This reaction produces heat and can even cause explosions if not handled carefully.
- Oxidation: When exposed to air, lithium tarnishes quickly, forming a dull, gray surface coating.
Solid lithium is widely used in batteries, as it’s stable enough to store and transfer energy efficiently.
Liquid Lithium
Lithium melts into a liquid when heated to 180.5°C (356.9°F). This state is less common but extremely useful in high-temperature applications. For example:
- Nuclear reactors: Liquid lithium is a coolant because it can absorb and transfer heat effectively.
- Metal alloys: Liquid lithium can be mixed with other metals to create lightweight and strong materials.
However, working with liquid lithium requires careful handling due to its high reactivity.
Gaseous Lithium
Lithium’s gaseous state is rare because it requires extreme heat. At a boiling point of 1,342°C (2,448°F), lithium vaporizes into a gas.
While people don’t commonly use this state in everyday applications, researchers study gaseous lithium to understand its behavior at an atomic level. This knowledge helps improve technologies like batteries and nuclear reactors.
Part 4. What makes lithium unique?
Lithium is not your average metal. It has several unique properties that set it apart:
- Lightweight: Manufacturers use lithium in products where weight is a concern, like aerospace materials and batteries because it is so light.
- High reactivity: Lithium reacts quickly with other substances as an alkali metal. This makes it useful in chemical reactions but also requires careful storage.
- High energy density: Lithium has one of the highest energy densities of any element, which is why it’s perfect for battery use.
- Thermal conductivity: Engineers use lithium in heat transfer applications because it conducts heat efficiently.
These properties make lithium a “super-metal” essential for modern technology.
Part 5. Why is lithium important in batteries?
Lithium-ion batteries are everywhere—your smartphone, electric car, laptop, and even renewable energy storage systems. But why is lithium so perfect for batteries? Let’s break it down:
- Lightweight: Lithium’s low atomic mass allows manufacturers to create lightweight, portable batteries.
- High energy storage: Lithium-ion batteries can store large amounts of energy in a small space, making them ideal for compact devices.
- Rechargeable: Unlike traditional batteries, users can recharge lithium-ion batteries hundreds of times without losing much capacity.
- Long lifespan: With proper care, lithium-ion batteries last longer than other types of batteries.
These batteries are also crucial for the transition to renewable energy. Solar panels and wind turbines produce power intermittently, but lithium-ion batteries can store that energy for later use.
Part 6. Where is lithium found?
Lithium is not found in its pure form in nature because it reacts too quickly with other elements. Instead, people extract it from:
- Hard rock minerals: Minerals like spodumene and petalite contain lithium. Miners extract these from the Earth’s crust.
- Brine pools: People extract lithium-rich brine from Chile, Argentina, and Bolivia salt flats. Due to its abundant reserves, this region is called the “Lithium Triangle.”
- Seawater: Lithium exists in trace amounts in seawater, but extracting it economically remains challenging.
Australia is currently the largest producer of lithium, followed by Chile and China.
Part 7. How is lithium processed?
After extraction, people must process lithium before they can use it. Here’s how it works:
- Mining or brine extraction: Lithium is either mined from rocks or pumped out of brine pools.
- Purification: The raw lithium is purified to remove impurities and converted into lithium carbonate or hydroxide.
- Manufacturing: These purified compounds create batteries, ceramics, lubricants, and other products.
Processing lithium is energy-intensive, so researchers are working on more sustainable methods.
Part 8. Is lithium dangerous?
Lithium has many benefits, but it also comes with risks:
- Reactivity: Lithium reacts violently with water, releasing hydrogen gas and heat. This can lead to explosions if not handled carefully.
- Health concerns: Prolonged exposure to lithium dust or compounds can irritate the skin, eyes, or respiratory system.
- Environmental impact: Mining lithium can harm local ecosystems and deplete water resources.
Despite these dangers, lithium is safe when handled and stored correctly.
Part 9. What are the other uses of lithium?
Lithium is best known for its role in batteries, but it has many other applications:
- Medicine: Doctors use lithium compounds to treat bipolar disorder and depression, helping stabilize mood swings.
- Glass and ceramics: Lithium improves the strength and thermal resistance of glass and ceramics, making them durable and heat-resistant.
- Lubricants: Industries use lithium-based greases widely in cars and machinery.
- Aerospace: Lithium alloys are used in aircraft and spacecraft to reduce weight without compromising strength.
These diverse uses highlight lithium’s versatility.
Part 10. FAQs
-
Is lithium always a solid at room temperature?
Yes, lithium is a solid at room temperature under normal pressure. However, it can become a liquid or gas when exposed to high heat. -
Why does lithium react with water?
Lithium is an alkali metal that reacts with water. When it comes into contact with water, it releases hydrogen gas and forms lithium hydroxide. This reaction generates heat and can be dangerous. -
Where is lithium most commonly found?
People most commonly find lithium in brine pools and hard rock deposits. The largest reserves lie in South America’s Lithium Triangle, Australia, and China. -
Can lithium be recycled?
Yes, people can recycle lithium from used batteries. However, experts find the process complex and are still improving it to make it more efficient and cost-effective. -
Is lithium toxic to humans?
Lithium is not toxic in small amounts; people even use it in medicine. However, high doses or prolonged exposure to lithium compounds can be harmful.
Related Tags:
More Articles

Battery Load Test: A Comprehensive Guide
Step-by-step battery load test guide for car, solar & industrial use. Learn how to load test a battery, interpret voltage charts, and avoid common mistakes.
The Comprehensive Guide to Battery Balancing and Battery Balancer
Discover how battery balancers improve lithium battery performance, lifespan, and safety. Learn types, functions, and tips to choose the right balancer.
What Is the Best Voltage for a Chainsaw Battery?
Compare 12V-80V chainsaw batteries for light pruning, medium firewood, and professional cutting. See best battery chainsaw with runtime charts and safety tips.
Lithium VS. Alkaline Batteries: A Comprehensive Comparison
Lithium batteries last 3–7× longer than alkaline and perform better in cold weather. Compare lifespan, cost, safety, and best uses to choose the right battery.
Comparing Lithium-Sulfur and Lithium-Ion Batteries: Which is Right for You?
Compare lithium-sulfur (Li-S) and lithium-ion batteries on energy, lifespan, cost, safety, and applications. Best choice for drones, EVs, and electronics.