- Part 1. What is an anode?
- Part 2. How does battery chemistry affect anode charge?
- Part 3. Is the anode positive or negative in alkaline batteries?
- Part 4. Is the anode positive or negative in lithium-ion batteries?
- Part 5. Is the anode positive or negative in lead-acid batteries?
- Part 6. How do rechargeable batteries differ from non-rechargeable ones?
- Part 7. What happens during charging?
- Part 8. What are some common misconceptions about battery terminals?
- Part 9. FAQs
One of the most fundamental questions when exploring the world of batteries is whether the anode is positive or negative in different battery types. This distinction is crucial for understanding how batteries function and how they power our devices. The anode plays a pivotal role in the electrochemical reactions that occur within batteries, and its charge can vary depending on the battery chemistry and its operational state.
This comprehensive article will delve into the nature of anodes across various battery types, clarify their functions, and provide clear answers regarding their charge. By the end, you will thoroughly understand anodes and their significance in battery technology.
Part 1. What is an anode?
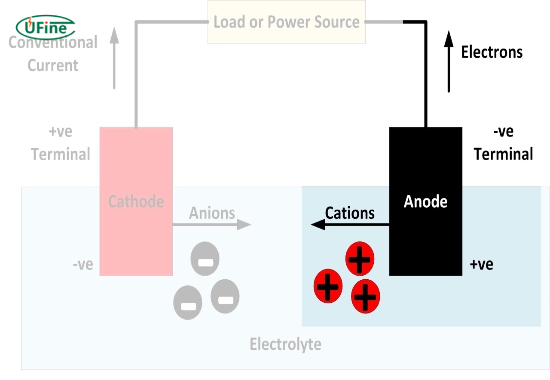
An anode is one of two electrodes in a battery where oxidation occurs during electrochemical reactions. In simpler terms, it is the site where electrons leave the battery and flow into the external circuit. The charge of the anode can be either positive or negative, depending on the type of battery and its state of operation.
Key Functions of an Anode
- Electron Flow: The anode’s primary function is to facilitate the flow of electrons from the battery to the external circuit.
- Oxidation Reaction: At the anode, oxidation occurs, meaning that electrons are released as a substance loses electrons during a chemical reaction.
- Electrolyte Interaction: The anode interacts with the electrolyte to enable ion movement, essential for maintaining charge balance within the battery.
Understanding these functions helps clarify why knowing whether an anode is positive or negative is critical for anyone working with batteries or electronic devices.
Part 2. How does battery chemistry affect anode charge?
The charge at the anode depends significantly on the battery’s chemistry. Different types of batteries utilize various chemical reactions that dictate whether the anode is positive or negative.
Common Battery Types and Their Anodes
- Alkaline Batteries: In alkaline batteries, zinc serves as the anode and is negatively charged during discharge.
- Lithium-Ion Batteries: Graphite is typically used as the anode in lithium-ion batteries. When discharging, it acts as a negative electrode.
- Lead-Acid Batteries: Lead dioxide (PbO2) is the positive terminal during discharge, while sponge lead (Pb) is the negative terminal.
Each type of battery has its unique chemistry that influences how it operates, and its components interact.
Part 3. Is the anode positive or negative in alkaline batteries?
In alkaline batteries, the anode is negative. During discharge, zinc undergoes oxidation at this electrode, releasing electrons that flow through the circuit to power devices. The chemical reaction can be summarized as follows:
Zn → Zn²⁺ + 2e⁻
This indicates that zinc loses electrons (is oxidized), confirming that it functions as a negative electrode. Understanding this process helps clarify why alkaline batteries are commonly used in household devices like remote controls and toys.
Part 4. Is the anode positive or negative in lithium-ion batteries?
In lithium-ion batteries, the anode is also negative when discharging. The primary material used for this electrode is graphite. Lithium ions move from cathode to anode during charging and intercalate into graphite layers. The reaction at the anode can be represented as:
Li⁺ + e⁻ + C → LiC₆
This shows lithium ions gain electrons (reduction) at this electrode when charging and lose them (oxidation) when discharging. Lithium-ion batteries are widely used due to their high energy density and ability to recharge quickly.
Part 5. Is the anode positive or negative in lead-acid batteries?
In lead-acid batteries, the anode is negative during discharge. The sponge lead (Pb) acts as this electrode, while lead dioxide (PbO2) is the cathode. The oxidation reaction at the anode can be expressed as:
Pb + SO₄²⁻ → PbSO₄ + 2e⁻
This indicates that lead loses electrons (is oxidized), confirming its role as a negative electrode. Lead-acid batteries are commonly used in automotive applications for their reliability and cost-effectiveness.
Part 6. How do rechargeable batteries differ from non-rechargeable ones?
Rechargeable and non-rechargeable batteries differ significantly in their chemical processes and electrode behavior.
Non-Rechargeable Batteries
- Permanent Reactions: Non-rechargeable batteries cannot be restored to their original state once discharged.
- Fixed Anodes: The charge state of their anodes remains constant throughout their life cycle.
Rechargeable Batteries
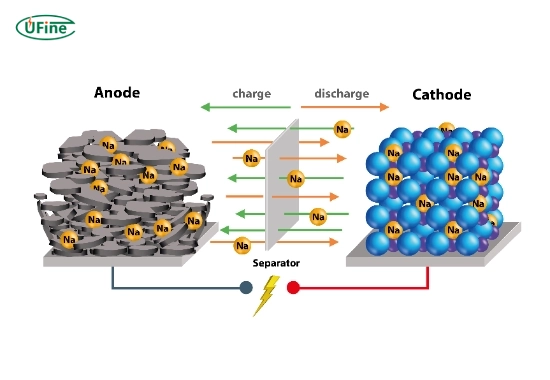
- Reversible Reactions: Rechargeable batteries allow for reversible electrochemical reactions.
- Variable Anodes: The charge state of their anodes can change depending on whether they are charging or discharging.
This distinction is essential for consumers when choosing between different types of batteries for specific applications.
Part 7. What happens during charging?
During charging, an external power source forces electrons back into the battery. This process reverses oxidation at the anode and restores chemical potential energy within the battery.
Charging Process Overview
- Lithium-Ion Batteries: Lithium ions move from cathode to anode and get stored within graphite layers.
- Lead-Acid Batteries: Lead sulfate converts to lead dioxide at the cathode while lead regenerates at the anode.
Understanding how charging works provides insight into why proper charging techniques are vital for maximizing battery life and performance.
Part 8. What are some common misconceptions about battery terminals?
Many people need clarification on battery terminals and their respective charges due to the varying terminologies used across different contexts.
Misconceptions Clarified
- Positive vs Negative Labels: Some may think that “positive” always refers to higher potential; however, this may not hold in specific contexts like discharging versus charging states.
- Terminal Functionality: It’s essential to understand that while one terminal may be labeled positive or negative based on conventional definitions, its role can change depending on whether it’s being charged or discharged.
These misconceptions can lead to improper handling or usage of batteries if not appropriately clarified.
Part 9. FAQs
-
What is a battery terminal?
A battery terminal is a conductive point where connections are made to draw or supply power to a battery. -
Why does it matter if an anode is positive or negative?
The charge of an anode determines how it interacts with other components within a circuit and affects overall device performance. -
Can you use different types of batteries interchangeably?
No, using different types of batteries interchangeably can damage devices due to differences in voltage and chemistry. -
How do I know when my battery needs replacing?
Signs include decreased performance, physical swelling, leakage, or failure to hold a charge over time. -
Are there safety concerns with rechargeable batteries?
Yes, improper handling can lead to overheating or even explosions; always follow manufacturer guidelines for charging and usage.
Related Tags:
More Articles

Battery Load Test: A Comprehensive Guide
Step-by-step battery load test guide for car, solar & industrial use. Learn how to load test a battery, interpret voltage charts, and avoid common mistakes.
The Comprehensive Guide to Battery Balancing and Battery Balancer
Discover how battery balancers improve lithium battery performance, lifespan, and safety. Learn types, functions, and tips to choose the right balancer.
What Is the Best Voltage for a Chainsaw Battery?
Compare 12V-80V chainsaw batteries for light pruning, medium firewood, and professional cutting. See best battery chainsaw with runtime charts and safety tips.
Lithium VS. Alkaline Batteries: A Comprehensive Comparison
Lithium batteries last 3–7× longer than alkaline and perform better in cold weather. Compare lifespan, cost, safety, and best uses to choose the right battery.
Comparing Lithium-Sulfur and Lithium-Ion Batteries: Which is Right for You?
Compare lithium-sulfur (Li-S) and lithium-ion batteries on energy, lifespan, cost, safety, and applications. Best choice for drones, EVs, and electronics.