- Part 1. What is lithium-ion battery internal resistance?
- Part 2. Lithium battery internal resistance and battery types
- Part 3. Factors influencing lithium-ion battery internal resistance
- Part 4. How does internal resistance affect performance?
- Part 5. Internal resistance and battery Life
- Part 6. How to calculate & find battery internal resistance? [3 Proven methods]
- Part 7. How to measure battery internal resistance?
- Part 8. How to reduce lithium battery internal resistance?
- Part 9. Lithium battery internal resistance: Top FAQs
Lithium-ion battery internal resistance is critical in determining battery performance, efficiency, and lifespan. Understanding what it is, how to measure it, and ways to reduce it can help optimize battery use for better energy output and longer life.
This guide will explore the factors influencing internal resistance, practical tips to lower it, methods for accurate measurement, and its effects on different battery types like lithium-ion, lead-acid, and NiMH. Whether you are a professional or a consumer, mastering this knowledge is key to maximizing battery efficiency and reliability.
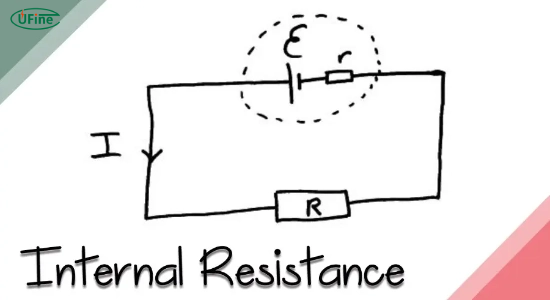
Part 1. What is lithium-ion battery internal resistance?
Ohmic Resistance
Lithium Ion Battery internal resistance encompasses various elements hindering the current flow within the battery. Ohmic resistance, a fundamental component, represents the inherent opposition within the battery’s components. This resistance arises due to the physical properties of the battery materials, including the electrodes, electrolytes, and separators. Ohmic resistance is quantified in ohms and contributes to the voltage drop experienced during current flow.
Polarization Resistance
Another aspect of Lithium Ion Battery internal resistance is polarization resistance. This resistance arises due to the electrochemical processes occurring within the battery during charge and discharge cycles. Polarization resistance involves phenomena such as the movement of ions, charge transfer at electrode interfaces, and concentration polarization within the electrolyte. It impacts energy conversion efficiency within the battery and affects its overall Performance.
Part 2. Lithium battery internal resistance and battery types
Internal resistance varies significantly between battery types. Understanding these differences can help you select the right battery for specific applications.
Lithium-Ion Batteries
- Low Internal Resistance: Typically ranges between 10-50 milliohms, depending on capacity and design.
- High Efficiency: These batteries provide better voltage stability under load, making them ideal for high-performance devices like smartphones or EVs.
Lead-Acid Batteries
- Higher Resistance: Usually ranges between 100-300 milliohms.
- Slower Response: These batteries lose more energy to heat, making them less suitable for rapid charge-discharge cycles.
Nickel-Metal Hydride (NiMH) Batteries
- Moderate Resistance: Falls between lithium-ion and lead-acid batteries.
- Steady Performance: NiMH batteries perform well in moderate-load applications like cordless tools or cameras.
Key Takeaway
- Lithium-ion batteries offer the best balance of low internal resistance and efficiency, which is why they are preferred for most modern applications. However, lead-acid or NiMH batteries may still be viable options for low-cost or backup systems.
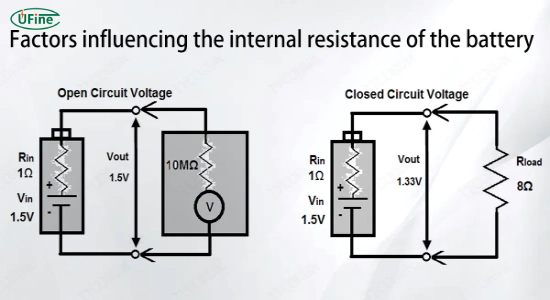
Part 3. Factors influencing lithium-ion battery internal resistance
Electrolyte
The composition and conductivity of the electrolyte significantly impact internal resistance. Electrolytes with higher ion conductivity reduce resistance, allowing smoother ion movement between electrodes. Conversely, less conductive electrolytes increase internal resistance, impeding ion flow and affecting battery performance.
Diaphragm
The diaphragm or separator within the battery influences internal resistance by controlling ion movement. Its permeability and structure impact the resistance by facilitating or restricting the passage of ions between electrodes. Optimized diaphragm design can lower resistance and enhance battery efficiency.
Collector
The material and design of the current collector affect internal resistance. Proper collector design ensures efficient electron transfer between electrodes, minimizing resistance. Materials with high conductivity, such as copper or aluminum, can reduce resistance by aiding electron flow.
Current
The magnitude and direction of the current passing through the battery affect internal resistance. Higher currents can lead to increased resistance due to factors like heat generation and changes in ion mobility within the battery’s components.
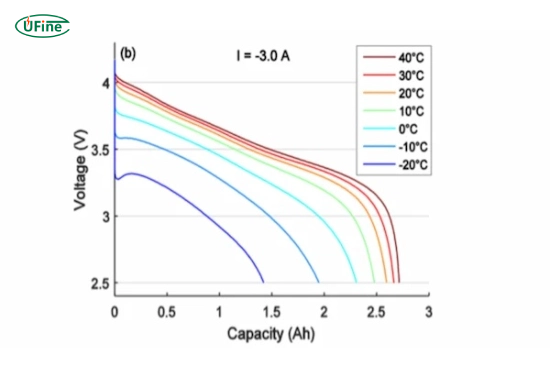
Temperature
Temperature variations play a critical role in internal resistance. Low temperatures increase resistance by restricting ion movement, impacting battery performance. Conversely, high temperatures might lower resistance but can accelerate degradation processes, affecting long-term resistance.
Manufacturing Process
The battery manufacturing process influences internal resistance. Factors like electrode thickness, material quality, assembly techniques, and quality control measures impact the uniformity of the battery’s components, subsequently affecting internal resistance. Well-optimized manufacturing processes can result in lower internal resistance and enhanced battery performance.
| Factor | Impact | Improvement Method |
|---|---|---|
| Electrolyte | Higher ion conductivity reduces resistance; lower conductivity impedes ion flow. | Use high-conductivity electrolytes to enhance ion movement. |
| Diaphragm | Controls ion movement; affects resistance based on permeability and structure. | Optimize diaphragm design for better ion flow and reduced resistance. |
| Collector | Impacts electron transfer efficiency; poor design increases resistance. | Use high-conductivity materials like copper or aluminum. |
| Current | Higher currents increase resistance due to heat and ion mobility changes. | Limit excessive currents and ensure balanced current flow. |
| Temperature | Low temperatures restrict ion movement; high temperatures accelerate degradation. | Maintain an optimal temperature range during operation. |
| Manufacturing Process | Impacts component uniformity; poor quality increases resistance. | Ensure high-quality materials and optimized assembly techniques. |
Internal Resistance by Battery Model (Reference Values)
| Battery Type | Typical IR Range | Common Applications |
|---|---|---|
| 18650 Lithium-ion | 20-80 mΩ | Laptops, Power tools |
| LiFePO4 (26650) | 2-20 mΩ | Solar storage, EVs |
| Lead-Acid (12V) | 100-300 mΩ | Automotive backup |
Key Insight: LiFePO4 batteries exhibit 50% lower internal resistance than standard lithium-ion, explaining their superior cycle life in high-demand applications like electric vehicles.
Part 4. How does internal resistance affect performance?
Voltage Output and Sag
Internal resistance significantly influences a battery’s ability to maintain a steady voltage output when powering a device. For instance, in a smartphone with a Lithium Ion Battery exhibiting high internal resistance, when the device experiences heavy usage (like gaming or video streaming), the battery voltage might sag noticeably, causing the device to shut down or prompt a low battery warning prematurely.
Efficiency and Energy Loss
Consider an electric vehicle (EV) powered by a lithium-ion battery with increased internal resistance. The higher resistance leads to energy loss as heat during charge and discharge cycles. This lost energy, instead of contributing to the vehicle’s propulsion, dissipates as heat, reducing the overall efficiency of the EV and decreasing its driving range per charge.
Capacity and Run Time
The adequate capacity diminishes in a laptop utilizing a Lithium Ion Battery with elevated internal resistance. The battery’s capacity decreases as the resistance increases, affecting the laptop’s run time between charges. This reduced usable capacity shortens the device’s operational duration, requiring more frequent recharging.
Heat Generation and Battery Health
Consider a power tool powered by a Lithium Ion Battery with increased internal resistance. The higher resistance causes more heat to be generated during high-demand tasks. This excessive heat accelerates battery degradation, reducing its lifespan. Over time, the tool’s battery loses its ability to hold a charge. It becomes prone to failure due to increased internal resistance-induced heat stress.
Internal Resistance Impact on Battery Performance
Part 5. Internal resistance and battery Life
As batteries age, their internal resistance increases, leading to reduced performance. The key reasons include:
- Material Degradation: Over time, the electrodes and electrolyte degrade, causing more resistance to ion movement.
- Increased Heat Generation: Higher resistance means more energy is lost as heat, which accelerates wear.
Impact on Capacity
Higher resistance lowers the battery’s usable capacity. For instance, a smartphone battery with increased resistance might show a full charge but drain quickly under heavy use.
Tips to Extend Battery Life
- Maintain Optimal Temperature: Store and use batteries at moderate temperatures (15°C to 30°C).
- Avoid Deep Discharge: Do not let the battery fully discharge; recharge when it drops to around 20-30%.
- Use Smart Chargers: High-quality chargers with built-in protections can help regulate charging and reduce heat.
Part 6. How to calculate & find battery internal resistance? [3 Proven methods]
-
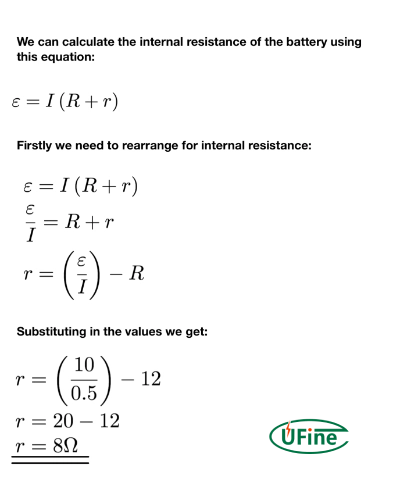
Voltage Drop Method (Most Accessible)
Equipment Needed: Digital multimeter + Load resistor
Steps:
- Measure open-circuit voltage (Voc)
- Apply known load (e.g., 10Ω resistor)
- Measure voltage under load (Vload)
- Calculate current: I = Vload / Rload
- Calculate internal resistance: Rinternal = (Voc – Vload) / I
Accuracy: ±10% | Best for: Quick field tests
-
AC Impedance Spectroscopy (Lab Precision)
Equipment Needed: Battery impedance analyzer (e.g., Hioki BT4560)
Steps:
- Connect battery to analyzer terminals
- Set frequency range (typically 0.1Hz-1kHz)
- Apply small AC signal (10-50mV amplitude)
- Measure impedance at multiple frequencies
- Find internal resistance at highest frequency point
Accuracy: ±1% | Best for: R&D and quality control
-
Pulse Discharge Method (Industrial Standard)
Equipment Needed: Programmable battery tester
Steps:
- Apply short current pulse (1-10 seconds)
- Measure instantaneous voltage drop (ΔV)
- Record current during pulse (I)
- Calculate: Rinternal = ΔV / I
- Repeat at different SOC levels
Accuracy: ±5% | Best for: Production line testing
Method Comparison Table
| Method | Cost | Time | Accuracy | Best Use Case |
|---|---|---|---|---|
| Voltage Drop | $ | 2-5 min | Medium | Field maintenance |
| AC Impedance | $$$ | 10-30 min | High | Laboratory R&D |
| Pulse Discharge | $$ | 1-5 min | High | Manufacturing QC |
Professional Tips for Accurate Measurement
- Temperature control: Always measure at 25±2°C (resistance changes 0.5-1%/°C)
- State of Charge: Test at 50% SOC for most consistent results
- Contact resistance: Clean terminals and use 4-wire Kelvin connection for professional setups
- Safety first: Never exceed battery’s maximum discharge current during testing
Part 7. How to measure battery internal resistance?
Use High-Quality Testing Devices
Reliable devices like Hioki Battery Testers or Fluke Precision Instruments can provide accurate internal resistance measurements. These tools measure resistance under different conditions, such as during charging or discharging. They are widely used in labs and industries for detailed analysis.
Step-by-Step Process
- Ensure Safety: Disconnect the battery from devices or systems to avoid short circuits.
- Measure Open Circuit Voltage: Use a multimeter to measure the battery voltage without a load.
- Apply a Load: Connect a known resistor or testing device to the battery.
- Record Voltage Drop: Measure the battery voltage while the load is applied.
- Calculate Resistance: Use Ohm’s Law (R = ΔV/ΔI), where ΔV is the voltage difference and ΔI is the current change.
Avoid Risks
- Ensure the load applied is within the safe current limits of the battery.
- Avoid prolonged testing at high currents to prevent overheating or damage.
Part 8. How to reduce lithium battery internal resistance?
Optimize Charging Methods
Using the right charging method can lower internal resistance and improve battery performance. A constant current charging method is one of the best options. This method prevents excessive heat build-up, which reduces the stress on battery materials and helps maintain lower resistance levels. Avoid fast charging unless necessary, as it generates more heat and increases resistance over time.
Choose High-Quality Materials
Batteries made with high-grade materials tend to have lower resistance. Look for batteries with advanced electrode materials, such as high-conductivity carbon additives or improved lithium compounds. Good thermal management systems can also help reduce heat build-up, keeping resistance at optimal levels. Properly designed batteries with efficient cooling mechanisms improve long-term performance.
Part 9. Lithium battery internal resistance: Top FAQs
What is normal internal resistance for lithium batteries?
Typical internal resistance ranges:
- Consumer Li-ion (18650): 20-80 mΩ
- EV Batteries: 0.5-2 mΩ per cell
- LiFePO4: 2-20 mΩ (50% lower than standard Li-ion)
What makes LiFePO4 internal resistance special?
LiFePO4 batteries have significantly lower resistance due to:
- Stable crystal structure reducing polarization
- Superior thermal stability minimizing heat impact
- Flatter discharge curve maintaining voltage
How to measure 18650 battery internal resistance?
Professional measurement method:
- Use battery analyzer (e.g., YR1035+)
- Set to 1kHz AC measurement mode
- Connect probes directly to cell terminals
- Read value when stable (20-80mΩ range)
How to reduce LiFePO4 internal resistance?
Effective reduction techniques:
- Maintain 20-30°C operating temperature
- Limit fast charging to <80% capacity
- Apply pulse charging technology
- Store at 40-60% state-of-charge
Why does lithium battery resistance increase over time?
Primary causes of resistance growth:
- SEI layer growth on anode (adds 5-15mΩ/year)
- Electrolyte decomposition at high temps
- Current collector corrosion
- Mechanical stress from cycling
Related Tags:
More Articles

12V STD vs 12V AGM: Meaning, Differences, and Which Is Better
Understand what STD and AGM batteries mean, their key differences, and which 12V battery fits your needs best in 2026.
Battery Reconditioning Explained: A Comprehensive Guide
Learn what battery reconditioning is, how it works, how long it takes, and when reconditioning chargers are used for lead-acid and lithium-ion batteries.
Recommended 10 Best Batteries For Smoke Detectors
Compare the best batteries for smoke detectors in 2026. See which 9V lithium and alkaline batteries last longest and perform best in smoke alarms.
Triple A Battery Voltage: Everything You Need to Know
How many volts is a AAA battery? See a clear AAA battery voltage chart, compare alkaline, lithium and NiMH cells, and learn how voltage affects device use.
What Size Battery for Digital Thermometer: The Complete Guide
What size battery does a digital thermometer use? See common sizes like AAA, AA, CR2032, and LR41, plus tips to choose the right battery and extend lifespan.