Lithium-ion battery structure powers many of our everyday devices. This article will explore their key components, how they work, and their different structures. We’ll also look at their design, manufacturing process, and safety. Finally, we’ll discuss the latest innovations in lithium-ion battery technology.
Part 1. What is the structure of a lithium-ion battery?
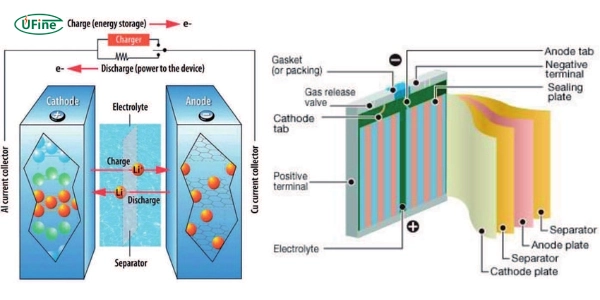
Lithium-ion batteries have several vital components that store and release energy. These components include the anode, cathode, electrolyte, and separator.
Battery Anode
The anode is a vital part of a lithium-ion battery. It stores the lithium ions when the battery is charged. The most common material used for the anode is graphite. Graphite is good because it can hold a lot of lithium ions. Some newer batteries use silicon in the anode because it can have even more lithium. The anode releases lithium ions when the battery is used, sending them through the electrolyte to the cathode.
- Materials: Graphite, silicon
- Functions: Stores lithium ions, releases ions during discharge
Battery Cathode
The cathode is the part of the battery that holds the lithium ions when the battery is not in use. It is usually made from a metal oxide. Common materials for the cathode include lithium cobalt oxide (LiCoO2), lithium iron phosphate (LiFePO4), and lithium nickel manganese cobalt oxide (LiNiMnCoO2). Each material has different strengths. For example, LiCoO2 is known for its high energy density, while LiFePO4 is known for its safety and long life.
- Materials: Lithium cobalt oxide, lithium iron phosphate, lithium nickel manganese cobalt oxide
- Functions: Holds lithium ions during discharge, releases ions during charging
Battery Electrolyte
The electrolyte in a lithium-ion battery is the medium that carries the lithium ions between the anode and cathode. It can be a liquid, gel, or solid. Liquid electrolytes are most common and are usually made of lithium salt in an organic solvent. Solid electrolytes are being developed for safety reasons because they are less likely to leak. The electrolyte must allow lithium ions to move freely but prevent electrons from passing through.
- Role: Carries lithium ions between anode and cathode
- Types: Liquid (lithium salt in organic solvent), gel, solid
Battery Separator
The separator is a thin, porous film that keeps the anode and cathode apart. This prevents the battery from short-circuiting. The separator allows lithium ions to pass through it while blocking electrons. It is usually made from polyethylene (PE) or polypropylene (PP). Some separators are made from a combination of these materials to improve safety and performance.
- Purpose: Prevents short-circuiting, allows ion flow
- Materials: Polyethylene, polypropylene
Part 2. How do lithium-ion batteries work?
The Electrochemical Process
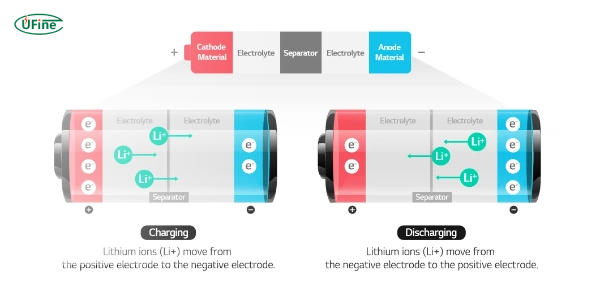
Lithium-ion batteries work through a process called electrochemistry. This involves chemical reactions that produce electricity. Lithium ions move from the cathode to the anode when the battery charges through the electrolyte. Electrons flow through an external circuit to balance the charge. When the battery discharges, the process reverses. Lithium ions return to the cathode, and electrons flow through the external circuit, creating an electric current.
- Charging: Lithium ions move to the anode, and electrons flow to balance the charge
- Discharging: Lithium ions return to the cathode, creating an electric current
Charge and Discharge Cycles
Lithium-ion batteries go through charge and discharge cycles to store and release energy. During charging, an external power source forces lithium ions into the anode. The lithium ions return to the cathode during discharging, providing power to devices. These cycles can happen many times before the battery’s performance starts to degrade. The number of cycles a battery can go through is called its cycle life.
- Charging: The power source moves ions to the anode
- Discharging: Ions return to the cathode, powering devices
- Cycle Life: Number of charge/discharge cycles before degradation
Energy Storage and Release Mechanism
Lithium-ion batteries’ energy storage and release mechanism involves the movement of lithium ions between the anode and cathode. When the battery is charging, the anode stores the lithium ions. This stored energy is released when the battery discharges as the ions return to the cathode. This movement of ions creates an electric current that powers devices like smartphones, laptops, and electric cars. The efficiency of this process makes lithium-ion batteries a popular choice for many applications.
- Storage: The anode stores lithium ions during charging
- Release: Ions move to the cathode during discharge, creating a current
- Applications: Powers devices like smartphones, laptops, and electric cars
Part 3. Design and configuration of lithium-ion batteries
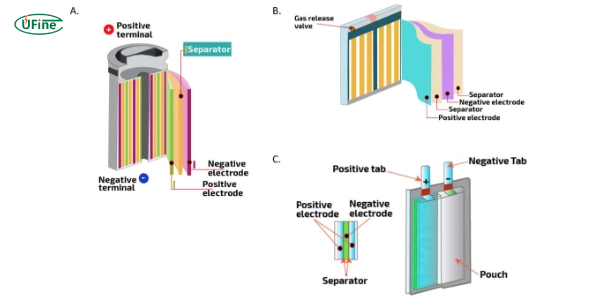
Cylindrical Cells
Cylindrical cells are one of the oldest and most widely used designs for lithium-ion batteries. They look like small metal cans and are very strong. These cells are used in many devices, from laptops to power tools. The design makes them easy to manufacture and calm, which helps with battery life and safety. However, their round shape can make it harder to pack them efficiently in some devices.
- Shape: Small metal cans
- Uses: Laptops, power tools, flashlights
- Advantages: Strong, easy to cool, simple to make
- Disadvantages: Harder to pack efficiently
Prismatic Cells
Prismatic cells are rectangular, easily fitting into slim and compact devices. They are commonly used in smartphones and tablets. These cells use space better than cylindrical cells but are more complex and costly to produce. The flat shape also makes it easier for heat to spread, improving performance.
- Shape: Rectangular
- Uses: Smartphones, tablets
- Advantages: Space-efficient, suitable for slim devices
- Disadvantages: More expensive to produce
Pouch Cells
Pouch cells are very flexible and lightweight. They have a soft outer shell that can be shaped to fit many different designs. This makes them ideal for portable electronics and electric vehicles. However, the soft shell makes them less durable than other types. They need careful handling and protection in the device design to avoid damage.
- Shape: Flexible, soft outer shell
- Uses: Portable electronics, electric vehicles
- Advantages: Lightweight, flexible shape
- Disadvantages: Less durable, needs careful handling
Part 4. The manufacturing process of lithium-ion batteries
The manufacturing process of lithium-ion batteries involves several key steps. First, the anode and cathode materials are mixed and coated onto metal foils. These foils are then dried, pressed, and cut into shapes. The anode, cathode, separator, and electrolyte are assembled into cells. The cells are filled with electrolytes and sealed. Finally, the batteries go through a formation process, where they are charged and discharged to ensure they work correctly.
- Steps: Mixing materials, coating foils, drying, pressing, cutting, assembling cells, filling with electrolyte, sealing
- Formation: Charging and discharging to ensure proper function
Part 5. Challenges in Lithium-ion Battery Structure
Lithium-ion batteries face several challenges in their structure. One major issue is thermal runaway, where the battery overheats and can catch fire. This is why battery management systems are crucial. Another challenge is capacity fading, where the battery’s ability to hold a charge decreases. Researchers are working on new materials and designs to address these issues. Balancing high energy density with safety and longevity remains a crucial challenge.
- Thermal Runaway: Overheating and potential fire
- Capacity Fading: Reduced ability to hold a charge over time
- Solutions: New materials, improved designs, better battery management systems
- Key Challenge: Balancing energy density, safety, and longevity
Part 6. Safety considerations in battery structure
Safety is a top priority in lithium-ion battery design. Separators must keep the anode and cathode apart to prevent short circuits. Overcharge protection is essential to stop the battery from overheating. Thermal management systems help keep the battery at a safe temperature. Manufacturers use various materials and technologies to enhance safety, like ceramic coatings on separators and advanced battery management systems. Regular testing and quality control are also vital to ensure battery safety.
- Separators: Prevent short circuits
- Overcharge Protection: Stops overheating
- Thermal Management: Maintains safe temperature
- Safety Enhancements: Ceramic-coated separators, advanced battery management systems
- Testing: Regular testing and quality control to ensure safety
Part 7. Innovations in lithium-ion battery structure
Solid-state Batteries
Solid-state batteries use a solid electrolyte instead of a liquid one. They are safer because they are less likely to leak or catch fire. These batteries can store more energy in a smaller space, which is great for electric cars and smartphones. Researchers are working to make them cheaper and more efficient.
Silicon Anodes
Silicon anodes can hold more lithium than traditional graphite ones. This means batteries can last longer and charge faster. But silicon expands and contracts, which can damage the battery. Scientists are finding ways to solve this problem and make silicon anodes more practical.
Lithium-sulfur Batteries
Lithium-sulfur batteries promise higher energy density at lower cost. They are suitable for electric vehicles and renewable energy storage. However, they need to last longer and be more stable. Researchers are working on improving their lifespan and reliability.
3D Battery Structures
3D battery designs pack more materials into a smaller space. This allows for faster charging and more power. They are still experimental but show potential for improving battery performance.
Part 8. FAQs
-
What are the main advantages of lithium-ion batteries over other types?
Lithium-ion batteries offer several advantages, including higher energy density, longer lifespan, and faster charging capabilities compared to traditional battery types like lead-acid or nickel-metal hydride (NiMH) batteries. -
Are lithium-ion batteries recyclable?
Yes, lithium-ion batteries are recyclable. They contain valuable materials like lithium, cobalt, and nickel, which can be extracted and reused in new batteries or other products. Recycling helps reduce environmental impact and conserves natural resources. -
Can lithium-ion batteries catch fire?
While rare, lithium-ion batteries can catch fire or explode if damaged or improperly handled. This is usually due to thermal runaway, where internal heat builds uncontrollably. Manufacturers incorporate safety features like battery management systems and quality control measures to prevent this. -
How long do lithium-ion batteries last?
The lifespan of a lithium-ion battery varies depending on factors like usage patterns, temperature conditions, and charging habits. These batteries can last between 2 to 10 years or more on average, with newer technologies and proper care extending their longevity.
Related Tags:
More Articles
Paper Battery vs. Flexible Battery: What’s the Difference and Which Is Better?
Paper vs. flexible batteries: learn the key differences, benefits, and which power source fits best for wearables, sensors, and smart tech.
What to Know Before Buying a Tiny LiPo Battery for Your Project
Tiny LiPo batteries are powerful and compact. Learn how to choose the right one for your project with specs, safety, and charging tips.
Bloated LiPo Battery: Will It Explode?
Will a bloated LiPo battery explode? Discover the causes, risks, safety steps, and expert tips to avoid disaster and protect your gear. Must-read safety guide!
12V 100Ah Lithium Ion Battery Price: Full Guide
Learn about 12V 100Ah lithium-ion battery price, from cost ranges to best brands, hidden fees, and how to get the best deal. A must-read for smart buyers!
Resistance and Conductivity: What It Means for Your Lithium Batteries
Resistance and conductivity impact lithium battery performance, lifespan, and safety—learn how they work and why they matter.