- Part 1. What is a lithium thionyl chloride battery?
- Part 2. How does a lithium thionyl chloride battery work?
- Part 3. What is a lithium metal battery?
- Part 4. How does a lithium metal battery work?
- Part 5. Comparing lithium thionyl chloride battery and lithium metal battery
- Part 6. Which battery is best for your application?
- Part 7. FAQs
Lithium batteries have become the cornerstone of modern portable electronics, medical devices, and various industrial applications. Among the many types of lithium batteries, Lithium Thionyl Chloride (Li-SOCl2) batteries and Lithium Metal batteries stand out due to their unique characteristics and applications. This article delves into the differences between these two types of batteries, exploring their compositions, advantages, disadvantages, and applications.
Part 1. What is a lithium thionyl chloride battery?
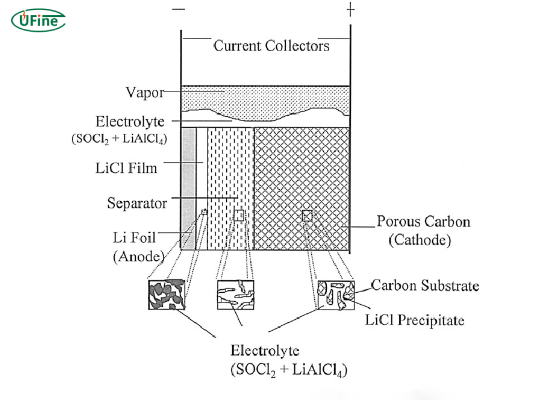
Lithium-thionyl chloride (Li-SOCl2) batteries are non-rechargeable batteries known for their high energy density and long shelf life. They use lithium metal as the anode and thionyl chloride as the cathode and electrolyte.
Composition of lithium thionyl chloride battery
The composition of Li-SOCl2 batteries includes:
- Anode: Lithium metal
- Cathode: Thionyl chloride (SOCl2)
- Electrolyte: Thionyl chloride also acts as the electrolyte
Advantages of lithium thionyl chloride battery
- High energy density: Li-SOCl2 batteries have one of the highest energy densities among primary batteries.
- Long shelf life: These batteries can have a shelf life of up to 10-20 years.
- Wide temperature range: They operate effectively in a broad temperature range from -55°C to +85°C.
- Low self-discharge rate: The rate is meager, less than 1% per year.
Disadvantages of lithium thionyl chloride battery
- Non-rechargeable: You cannot recharge the battery once it depletes its energy.
- Safety concerns: Thionyl chloride is a highly reactive and toxic substance.
- Cost: These batteries can be more expensive compared to other primary batteries.
Applications of lithium thionyl chloride battery
Industries widely use Li-SOCl2 batteries in applications such as:
- Utility metering (electricity, gas, water meters)
- Military applications (communication devices)
- Medical devices (implants, monitoring devices)
- Remote monitoring systems (sensors, beacons)
Part 2. How does a lithium thionyl chloride battery work?
Li-SOCl2 batteries operate through a chemical reaction between lithium and thionyl chloride. Lithium ions move from the anode to the cathode when the battery is in use, where they react with thionyl chloride to produce energy.
Chemical reactions in lithium thionyl chloride battery
The main reactions involved are:
- Anode reaction: Li → Li⁺ + e⁻
- Cathode reaction: SOCl₂ + 4e⁻ → SO₂ + 2Cl⁻
- Overall reaction: 4Li + SOCl₂ → 4LiCl + SO₂ + Energy
Part 3. What is a lithium metal battery?
Lithium Metal batteries encompass a range of batteries that use lithium metal as the anode. These can include primary (non-rechargeable) and secondary (rechargeable) batteries.
Composition of lithium metal battery
The composition of Lithium Metal batteries generally includes:
- Anode: Lithium metal
- Cathode: Various materials can be used, such as manganese dioxide, sulfur, or oxygen
- Electrolyte: Can be liquid, gel, or solid-state
Advantages of lithium metal battery
- High energy density: Lithium metal batteries offer high energy density, making them suitable for high-energy applications.
- Lightweight: Lithium is one of the lightest metals, contributing to the battery’s overall weight.
- High voltage: These batteries can provide higher voltage outputs than other battery types.
Disadvantages of lithium metal battery
- Safety issues: Lithium metal is highly reactive, and the batteries can be prone to thermal runaway and fires.
- Limited rechargeability: Rechargeable lithium metal batteries are still under development, and current technology has limitations in cycling stability.
- Cost: Lithium metal batteries are typically more expensive due to the cost of lithium and the complexities in manufacturing.
Applications of lithium metal battery
Lithium Metal batteries find applications in high-energy-demand scenarios such as:
- Aerospace and defense (drones, satellites)
- High-end electronics (cameras, laptops)
- Medical devices (hearing aids, pacemakers)
- Backup power (uninterruptible power supplies)
Part 4. How does a lithium metal battery work?
Lithium Metal batteries function by moving lithium ions between the anode and cathode through the electrolyte. The specific reactions depend on the cathode material used.
Chemical reactions in lithium metal battery
For a general Lithium Metal battery with a metal oxide cathode, the reactions are:
- Anode reaction: Li → Li⁺ + e⁻
- Cathode reaction: MxOy + Li⁺ + e⁻ → LiMxOy
- Overall reaction: Li + MxOy → LiMxOy
Part 5. Comparing lithium thionyl chloride battery and lithium metal battery
Energy density
- Li-SOCl2 batteries have a higher energy density compared to most lithium metal batteries.
- Lithium Metal batteries can vary in energy density based on the cathode material.
Shelf life
- Li-SOCl2 batteries offer a longer shelf life, often up to two decades.
- Lithium Metal batteries typically have shorter shelf lives, especially the rechargeable variants.
Safety
- Li-SOCl2 batteries pose significant risks due to the reactivity of thionyl chloride.
- Lithium Metal batteries are also prone to safety issues, particularly thermal runaway.
Cost
- Li-SOCl2 batteries are more expensive due to their specialized applications and materials.
- Lithium Metal batteries vary in cost, with some configurations being more affordable.
Rechargeability
- Li-SOCl2 batteries are non-rechargeable.
- Lithium Metal batteries include both non-rechargeable and rechargeable types, though the latter are still developing.
Part 6. Which battery is best for your application?
Choosing between Li-SOCl2 batteries and Lithium Metal batteries depends on the specific needs of your application:
- For long-term, low-power applications like utility metering, Li-SOCl2 batteries are ideal.
- Lithium metal batteries may be more suitable for high-power, lightweight applications like drones or medical devices.
Part 7. FAQs
-
What is the main advantage of lithium thionyl chloride batteries?
Li-SOCl2 batteries have the highest energy density and extended shelf life among primary lithium batteries, making them ideal for long-term, low-power applications. -
Are lithium metal batteries rechargeable?
Some Lithium Metal batteries are rechargeable, but they are still under development and face challenges related to cycling stability and safety. -
What are the safety concerns with lithium thionyl chloride batteries?
Li-SOCl2 batteries contain thionyl chloride, a highly reactive and toxic substance, which poses significant safety risks, including the potential for leakage and fire. -
Which battery type is more cost-effective?
Cost-effectiveness depends on the application. Li-SOCl2 batteries are more expensive but offer long-term reliability. In contrast, Lithium Metal batteries vary in cost based on their specific configuration and application. -
Can lithium metal batteries be used in high-temperature environments?
Lithium metal batteries operate in various temperatures, but extreme temperatures can compromise their performance and safety. Li-SOCl2 batteries generally have a broader operational temperature range.
Related Tags:
More Articles

고방전 배터리는 높은 전류와 발열로 배터리수명이 줄어듭니다. 구조적 한계, 사용 패턴, 충전 습관 등 실제 수명 단축 요인을 체계적으로 분석했습니다.
Capacitor vs Battery: What is the Difference?
Capacitor vs battery explained in detail. Learn the difference between capacitor and battery in energy storage, charging speed, lifespan, and real applications.
18650 Battery vs AA: Which Is Better for Your Device?
Compare 18650 vs AA batteries in capacity, voltage, rechargeability, and applications. Learn which battery type fits high-drain or everyday devices.
What is the Difference Between Battery Cell, Battery Control Module, and Battery Pack?
Compare battery cells, modules, and packs. Learn functions, design differences, control modules, and selection tips for EV, ESS, and industrial use.
How to Prevent LiPo Battery Explosion?
Can LiPo batteries explode or catch fire? Learn key causes of LiPo battery fires and proven charging, storage, and handling tips to reduce explosion risk.