Metal-air batteries are revolutionizing the energy storage landscape, offering a unique and efficient solution for various applications. Understanding the intricacies of metal-air batteries is vital as the world shifts towards sustainable energy sources. This article delves into their workings, advantages, challenges, and comparisons with traditional lithium-ion batteries, providing a comprehensive overview of this innovative technology.
Part 1. What are metal air batteries?
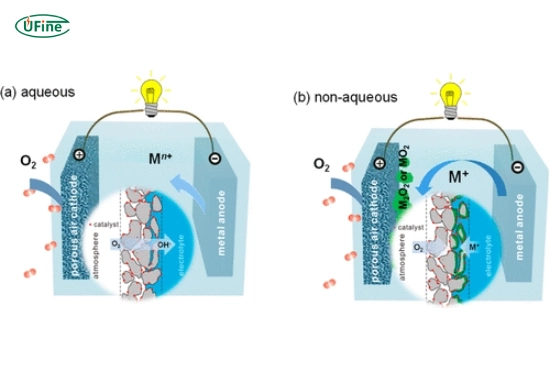
Metal air batteries are electrochemical cells that generate electricity through the oxidation of a metal, typically zinc or aluminum, in the presence of oxygen from the air. Unlike conventional batteries that rely on heavy materials and complex chemistries, metal air batteries leverage the abundant availability of oxygen, making them lighter and potentially more efficient.
How do they work?
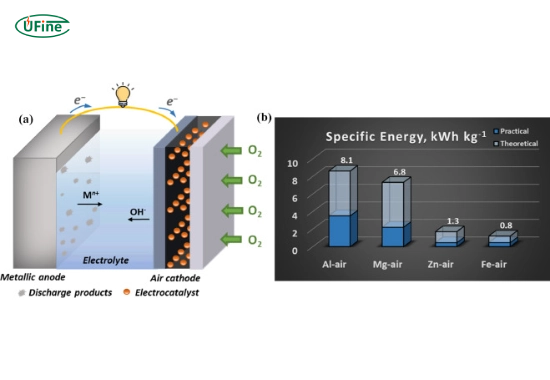
The essential operation of a metal air battery involves two electrodes: an anode made from a metal (like zinc) and a cathode that interacts with oxygen. When the battery discharges, the metal oxidizes at the anode, releasing electrons that flow through an external circuit to power devices. Simultaneously, oxygen from the air is reduced at the cathode. This process can be summarized in a simple equation:
- Metal+Oxygen→Electricity+Byproducts
Key components
- Anode: Typically made from metals like zinc or aluminum.
- Cathode: A porous material that allows oxygen to enter, facilitating electron transfer.
- Electrolyte: A medium that conducts ions between the anode and cathode.
Part 2. Advantages of metal air batteries
Metal air batteries come with several advantages that set them apart from traditional battery technologies:
- High energy density: Zinc-air batteries offer significantly higher energy density than lithium-ion batteries, making them ideal for applications where weight is critical. For instance, zinc-air batteries can achieve energy densities of around 300–400 Wh/kg.
- Lightweight: The use of lightweight materials and the reliance on ambient oxygen contribute to their overall reduced weight.
- Cost-effective: Metals like zinc are abundant and inexpensive compared to lithium or cobalt in conventional batteries.
- Environmental impact: Metal air batteries can be more sustainable, with less reliance on rare materials and potentially lower ecological footprints.
Part 3. Applications of metal air batteries
Metal air batteries have a wide range of applications due to their unique properties:
- Electric vehicles (EVs): Their high energy density makes them suitable for powering electric cars, potentially extending driving ranges significantly.
- Portable electronics: Lightweight and efficient energy storage can enhance the performance of smartphones and laptops.
- Grid storage: They can effectively store renewable energy generated from solar or wind sources.
Part 4. How do metal air batteries compare to lithium-ion batteries?
When comparing metal air batteries to lithium-ion batteries, several key differences emerge:
- Energy Density: Metal air batteries generally have higher energy densities than lithium-ion batteries. For example, zinc-air batteries can reach up to 400 Wh/kg, compared to lithium-ion batteries’ typical range of 150–250 Wh/kg.
- Weight: Metal-air batteries are lighter because they use ambient oxygen rather than carrying all reactants within the battery itself.
- Cost: The raw materials for metal air batteries (like zinc) are often cheaper and more abundant than those used in lithium-ion technology (such as cobalt).
- Cycle Life: Lithium-ion batteries typically have longer cycle lives (up to 2000 cycles) than many current metal air designs, which might only last around 100–300 cycles before performance degrades.
Comparison Table
| Feature | Metal Air Batteries | Lithium-Ion Batteries |
|---|---|---|
| Energy Density | 300–400 Wh/kg | 150–250 Wh/kg |
| Weight | Lighter due to ambient O2 | Heavier due to contained reactants |
| Cost | Generally lower | Higher due to rare materials |
| Cycle Life | 100–300 cycles | Up to 2000 cycles |
| Environmental Impact | Lower due to less rare materials | Higher due to mining impacts |
Part 5. What are the main types of metal air batteries?
Metal air batteries are classified based on the type of metal used in their anodes. Each type has unique characteristics, advantages, and challenges that make them suitable for different applications. Below, we explore the most common types of metal air batteries in detail.
1. Zinc-Air Batteries
Overview: Zinc-air batteries are the most widely studied and utilized metal air batteries. They use zinc as the anode and oxygen from the air as the cathode reactant.
Advantages:
- High Energy Density: They can achieve energy densities of around 300–400 Wh/kg, making them suitable for portable electronic devices and hearing aids.
- Cost-Effective: Zinc is abundant and inexpensive compared to other metals, contributing to lower production costs.
Challenges:
- Limited Rechargeability: While some designs allow recharging, zinc-air batteries suffer short cycle life due to byproduct buildup during discharge.
- Moisture Sensitivity: Performance can degrade in humid environments, leading to zinc anode corrosion.
2. Aluminum-Air Batteries
Overview: Aluminum-air batteries utilize aluminum as the anode material. Due to their high energy density, they are lovely for electric vehicles.
Advantages:
- Exceptional Energy Density: The theoretical energy density can reach 6–8 kWh/kg, significantly higher than lithium-ion batteries.
- Lightweight and Safe: Aluminum is lightweight and poses fewer safety risks than lithium-based systems.
Challenges:
- Non-Rechargeable: Most aluminum-air batteries are not rechargeable; they require new aluminum anodes once depleted, making them less practical for everyday use.
- Corrosion Issues: The aluminum anode can corrode quickly, especially in moisture, which limits its lifespan.
3. Magnesium-Air Batteries
Overview: Magnesium-air batteries use magnesium as the anode material. They offer a promising alternative due to magnesium’s abundance and low cost.
Advantages:
- High Theoretical Energy Density: Magnesium-air systems can achieve energy densities comparable to or exceeding those of zinc-air systems.
- Safety Profile: Magnesium is less reactive than lithium, reducing risks associated with battery failure.
Challenges:
- Electrolyte Limitations: The performance of magnesium-air batteries is heavily influenced by the choice of electrolyte; many aqueous electrolytes lead to rapid magnesium dissolution.
- Cycle Life Issues: Like zinc-air systems, they often face rechargeability and cycle life challenges.
4. Lithium-Air Batteries
Overview: Lithium-air batteries are still mainly experimental but have garnered significant interest due to their high theoretical energy density.
Advantages:
- Unmatched Energy Density Potential: Theoretical energy densities can reach up to 13,000 Wh/kg, far exceeding any other battery technology.
- Lightweight Design: Like other metal-air systems, they utilize ambient oxygen, resulting in lighter battery designs.
Challenges:
- Stability Issues: Lithium is highly reactive and can form unstable compounds during discharge and charge cycles, leading to performance degradation.
- Complexity of Rechargeability: Current lithium-air designs struggle with efficient recharging due to high overpotentials and side reactions during cycling.
5. Iron-Air Batteries
Overview: Iron-air batteries utilize iron as the anode material. They are being explored for large-scale energy storage applications.
Advantages:
- Low Cost and Abundance: Iron is one of the most abundant metals on Earth, making these batteries potentially very cost-effective.
- Good Energy Density Potential: They can achieve reasonable energy densities while environmentally friendly.
Challenges:
- Corrosion Problems: Iron tends to rust easily when exposed to moisture, which can limit its effectiveness in practical applications.
- Limited Cycle Life and Efficiency Issues: Current designs often need more efficiency and short cycle life than established battery technologies.
Comparison Table
| Type | Theoretical Energy Density | Rechargeable | Key Applications |
|---|---|---|---|
| Zinc-Air | 300–400 Wh/kg | Limited | Hearing aids, cameras |
| Aluminum-Air | Up to 6–8 kWh/kg | No | Electric vehicles |
| Magnesium-Air | Comparable to Zinc-Air | Limited | Potentially EVs |
| Lithium-Air | Up to 13,000 Wh/kg | Challenging | Experimental applications |
| Iron-Air | Reasonable | Limited | Large-scale storage |
Part 6. Challenges facing metal air batteries
Despite their advantages, several challenges hinder the widespread adoption of metal air batteries:
- Limited cycle life: Many metal air batteries suffer from limited rechargeability due to byproducts that can accumulate during operation.
- Moisture sensitivity: The performance can degrade in humid environments where water can interfere with chemical reactions.
- Manufacturing complexities: Producing these batteries at scale while maintaining quality and efficiency presents logistical challenges.
Part 7. FAQs
-
How long do metal air batteries last?
The lifespan varies significantly depending on design but typically ranges from 100 to 300 cycles for most current models. -
Are there any safety concerns with metal air batteries?
While generally safe, proper handling is essential as corrosion or byproduct buildup can pose risks if not managed correctly. -
Can metal air batteries be recharged?
Some designs allow for recharging; however, many current models face limitations in cycle life that affect their practicality for repeated use. -
What industries could benefit most from using metal air batteries?
Industries such as automotive (for electric vehicles), consumer electronics (like smartphones), and renewable energy sectors (for grid storage) could see significant benefits. -
How do researchers plan to overcome current limitations in metal air battery technology?
Ongoing research focuses on improving materials for better efficiency, developing hybrid systems that combine technologies, and enhancing manufacturing processes for scalability.
Related Tags:
More Articles
Learning Car Battery Voltage
Wondering what voltage a car battery operates at? Learn about the importance of car battery voltage, how to check it, common causes of voltage drops.
Understanding 9V Battery Amps
Discover how many amperes a 9V battery delivers, its capacity, discharge rate, and much more. Get all the details on current, types, lifespan, and alternatives.
Understanding the Difference Between AA and AAA Batteries
Wondering about the difference between AA and AAA batteries? Learn the key differences, similarities, and when to use each in this detailed guide.
Understanding AAA Battery Voltages in Simple Terms
Understand AAA battery voltages easily. Explore their uses in everyday devices. Get the facts you need to choose the right battery today!