Lithium hexafluorophosphate (LiPF₆) and sodium chloride (NaCl) are two compounds revolutionizing the energy storage landscape. LiPF₆ has long been the backbone of lithium-ion batteries, powering everything from smartphones to electric vehicles (EVs). Meanwhile, NaCl—a humble table salt—is emerging as a key player in sodium-ion batteries, offering a cheaper and more sustainable alternative for large-scale energy storage. This article dives deep into their chemistry, performance advantages, challenges, and the innovations driving their adoption in next-generation batteries. By understanding these materials, we can unlock the future of cleaner, more efficient energy systems.
Part 1. What is lithium hexafluorophosphate?
Lithium hexafluorophosphate (LiPF₆) is a lithium-based salt with the chemical formula LiPF₆. It is the primary electrolyte salt in nearly all commercial lithium-ion batteries. When dissolved in organic solvents like ethylene carbonate or dimethyl carbonate, LiPF₆ dissociates into lithium ions (Li⁺) and hexafluorophosphate anions (PF₆⁻). These free lithium ions shuttle between the cathode and anode during charging and discharging, enabling energy storage and release.
LiPF₆’s popularity stems from its balance of properties:
- High ionic conductivity (~10 mS/cm in carbonate solvents).
- Moderate electrochemical stability within the 3–4.5V voltage range.
- The ability to form a stable solid-electrolyte interphase (SEI) on graphite anodes prevents further electrolyte decomposition.
However, its synthesis involves reacting lithium fluoride (LiF) with phosphorus pentafluoride (PF₅) under controlled conditions. This process requires handling hazardous fluorine gas.
Part 2. Why is LiPF₆ the dominant electrolyte in lithium-ion batteries?
LiPF₆’s dominance is rooted in three critical factors:
1. Superior ionic conductivity
LiPF₆ dissolves efficiently in organic solvents, creating a conductive medium that allows lithium ions to rush. This ensures fast charging and high power output, which is critical for EVs and portable electronics.
2. Formation of a protective SEI layer
During the first charge cycle, LiPF₆ reacts with the graphite anode to form a thin, stable SEI layer. This layer acts as a barrier, preventing further electrolyte breakdown and extending the battery’s lifespan.
3. Compatibility with high-voltage cathodes
LiPF₆ remains stable at voltages up to 4.5V, making it suitable for high-energy cathodes like lithium nickel manganese cobalt oxide (NMC) and lithium cobalt oxide (LCO).
Drawbacks of LiPF₆:
- Thermal instability: At temperatures above 60°C, LiPF₆ decomposes into lithium fluoride (LiF) and phosphorus pentafluoride (PF₅), which further reacts with trace water to produce toxic hydrogen fluoride (HF).
- Moisture sensitivity: Even tiny amounts of water (≥50 ppm) can trigger degradation, necessitating ultra-dry manufacturing environments.
Part 3. Emerging alternatives to LiPF₆ and their limitations
While LiPF₆ remains irreplaceable in most applications, researchers are exploring alternatives to address its shortcomings:
Lithium bis(trifluoromethane sulfonyl)imide (LiTFSI)
Advantages: Higher thermal stability (>100°C) and moisture tolerance.
Drawbacks: Corrodes aluminum current collectors at voltages above 3.8V, limiting use in high-voltage systems.
Lithium bis(oxalate)borate (LiBOB)
Advantages: Forms a robust SEI layer and suppresses gas formation.
Drawbacks: Low solubility in carbonate solvents, reducing ionic conductivity.
Lithium difluoro(oxalato)borate (LiDFOB)
A hybrid of LiPF₆ and LiBOB, LiDFOB offers improved thermal stability but remains costly for mass production.
Why LiPF₆ still wins: No alternative matches its cost-performance balance. For instance, LiTFSI costs ~5x more than LiPF₆, making it impractical for consumer electronics.
Part 4. Sodium chloride’s role in battery electrolytes
Sodium chloride (NaCl), commonly known as table salt, is gaining traction as an electrolyte material for sodium-ion batteries. Unlike LiPF₆, NaCl dissolves in water, enabling aqueous electrolytes that are non-flammable and environmentally benign.
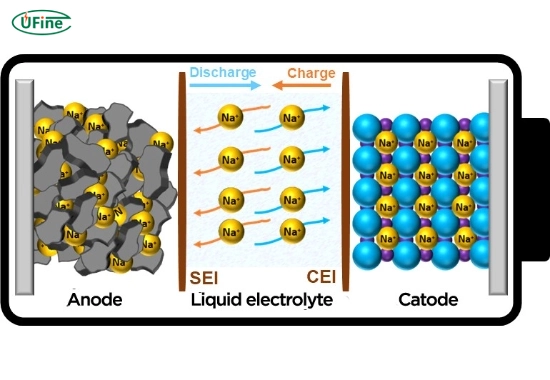
How NaCl works in sodium-ion batteries
- NaCl dissociates into sodium ions (Na⁺) and chloride ions (Cl⁻) in water during charging.
- Na⁺ ions migrate to the cathode (e.g., sodium vanadium phosphate) and anode (e.g., hard carbon), storing energy through redox reactions.
- The process reverses during discharge, releasing stored energy.
Advantages of NaCl:
- Ultra-low-cost: Sodium is 500x more abundant than lithium, and NaCl can be sourced from seawater.
- Safety: Water-based electrolytes eliminate fire risks associated with organic solvents.
- Sustainability: NaCl is non-toxic and more straightforward to recycle than LiPF₆.
Challenges:
- Low energy density: Sodium ions are more extensive (1.02 Å vs. Li’s 0.76 Å), leading to slower diffusion and reduced capacity.
- Limited voltage window: Aqueous systems are restricted to ~2V due to water electrolysis at higher voltages.
Part 5. LiPF₆ vs. NaCl: A detailed performance comparison
Cost and availability
| Factor | LiPF₆ | NaCl |
|---|---|---|
| Raw material cost | ~$50/kg (lithium carbonate) | ~$0.10/kg (from seawater) |
| Global reserves | 22 million metric tons (lithium) | Virtually unlimited (sodium) |
| Supply chain risks | Geopolitical tensions (e.g., South America’s Lithium Triangle) | Minimal |
Electrochemical performance
| Parameter | LiPF₆ (organic) | NaCl (aqueous) |
|---|---|---|
| Ionic conductivity | 10 mS/cm | 1–2 mS/cm |
| Voltage window | 3–4.5V | 1.5–2.5V |
| Cycle life | 500–1,000 cycles | 200–500 cycles (current R&D) |
| Energy density | 250–300 Wh/kg (NMC/graphite) | 100–150 Wh/kg (prototypes) |
Environmental impact
LiPF₆: Production involves toxic fluorine gas, and improper disposal releases HF, which contaminates soil and water.
NaCl: Biodegradable and poses minimal environmental risks, but sodium extraction requires energy-intensive processes.
Part 6. Can NaCl-based batteries compete with LiPF₆ systems?
Short answer: Not yet—but they fill critical gaps.
Where NaCl excels:
- Grid-scale energy storage: Sodium-ion batteries with NaCl electrolytes are ideal for storing solar/wind energy due to their low cost and safety. Companies like CATL plan to deploy them by 2025.
- Stationary applications: Fire-safe aqueous systems are suited for homes, hospitals, and data centers.
Where LiPF₆ dominates:
- High-energy applications: EVs, drones, and smartphones require the high energy density of lithium-ion batteries.
- Hybrid solutions: Researchers are exploring dual-ion systems where LiPF₆ powers high-performance devices while NaCl handles large-scale storage.
Part 7. Innovations enhancing LiPF₆ electrolytes
To extend LiPF₆’s lifespan and safety, scientists are pursuing:
1. Additive engineering
Hexamethyldisilazane (HMDS): Scavenges trace water and HF, improving thermal stability.
Fluoroethylene carbonate (FEC): Strengthens the SEI layer on silicon anodes, enabling higher-capacity batteries.
2. Solid-state hybrids
Mixing LiPF₆ with solid electrolytes like LLZO (Li₇La₃Zr₂O₁₂) reduces flammability and enhances high-temperature performance.
3. Advanced purification
Reducing water content below 10 ppm during manufacturing minimizes decomposition.
Part 8. Breakthroughs in NaCl electrolyte engineering
To make NaCl viable for broader use, recent advancements include:
1. Water-in-salt electrolytes (WiSE)
By supersaturating water with NaCl (up to 21 mol/kg), researchers achieve a 2.5V voltage window—close to non-aqueous systems. This approach also reduces water activity, slowing corrosion.
2. Organic solvent blends
Mixing NaCl with solvents like propylene carbonate boosts ionic conductivity to 5 mS/cm, rivaling early lithium-ion electrolytes.
3. Dual-salt systems
Combining NaCl with salts like sodium bis(fluorosulfonyl)imide (NaFSI) improves cycling stability. For example, a 2023 study showed a 400-cycle lifespan for a NaCl/NaFSI hybrid electrolyte.
Part 9. Environmental and safety trade-offs
LiPF₃: High performance, high risk
Toxicity: HF emissions during fires pose severe health risks, including lung damage.
Recycling hurdles: Only 5% of LiPF₆ is recycled due to complex separation from organic solvents.
NaCl: Eco-friendly but energy-intensive
Green credentials: NaCl electrolytes are non-toxic and quickly neutralized.
Carbon footprint: Sodium production via electrolysis consumes ~10 kWh/kg, higher than lithium’s 5 kWh/kg.
Part 10. The future: Solid-state and hybrid batteries
Solid-state LiPF₆ systems
Replacing liquid electrolytes with solid LiPF₆-based ceramics (e.g., Li₃PS₄) could enable safer, denser batteries. Toyota aims to commercialize these by 2030.
Solid-state NaCl batteries
Using clay or polymer matrices to immobilize NaCl electrolytes could create ultra-cheap batteries for home storage.
Hybrid lithium-sodium systems
Combining LiPF₆ and NaCl in separate cells within a single battery pack could optimize cost and performance.
Part 11. FAQs
Why can’t sodium chloride replace lithium hexafluorophosphate in current batteries?
NaCl cannot replace LiPF₆ because sodium ions are chemically incompatible with lithium-ion battery electrodes. Their larger size causes mechanical stress, leading to rapid capacity fade.
Is lithium hexafluorophosphate flammable?
LiPF₆ itself isn’t flammable, but its organic solvent electrolytes (e.g., ethylene carbonate) can ignite at high temperatures.
What is the main obstacle for NaCl in sodium-ion batteries?
The low ionic conductivity of aqueous NaCl electrolytes (~1–2 mS/cm vs. LiPF₆’s 10 mS/cm) limits charge/discharge speeds and energy density.
Are there any commercial products using NaCl electrolytes?
As of 2024, no—most are in the pilot stage. CATL’s first NaCl-based sodium-ion battery is expected to launch in 2025 for grid storage.
Can LiPF₆ and NaCl be recycled together?
No. Due to their differing chemistries, they require separate recycling processes. LiPF₆ recycling focuses on recovering lithium, while NaCl systems prioritize sodium and water treatment.
Related Tags:
More Articles

How to Choose the Best Floor Scrubber Battery for Commercial Cleaning?
Selecting the ideal floor scrubber battery ensures a long runtime, rapid charging, and minimal maintenance for efficient commercial cleaning operations.
Battery for Blower vs Battery for Leaf Vacuum: Which One Should You Choose?
Battery for blower vs leaf vacuum—learn the key differences in power, fit, and runtime to choose the right battery for your outdoor tool needs.
How to Choose the Right Battery for Blower?
Choosing the right blower battery? Consider voltage, capacity, chemistry & usage. This guide helps match the best battery for peak performance.
How to Choose the Best Insulated Battery Box for Lithium Batteries?
Choosing the Best Insulated Battery Box for Lithium Batteries? Discover key factors such as size, material, and safety for optimal protection and performance.
7 Critical Elements on a Lithium Battery Shipping Label
What must be on a lithium battery shipping label? Learn 7 key elements to ensure safety, legal compliance, and correct handling across all transport modes.