Zinc air batteries are a unique type of battery that utilizes the chemical reaction between zinc and oxygen from the air to generate electricity. This guide will delve into the intricacies of zinc air batteries, covering their composition, advantages, applications, and challenges.
Part 1. What is a zinc-air battery?
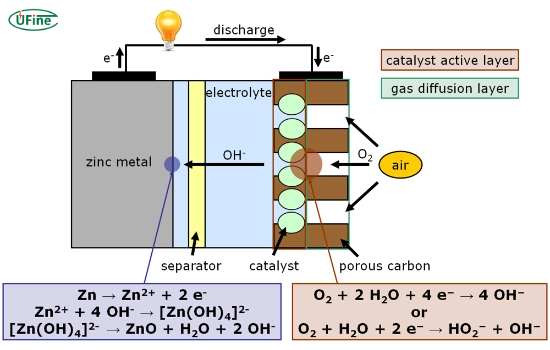
A zinc air battery is an electrochemical cell that converts chemical energy into electrical energy through zinc oxidation and oxygen reduction. These batteries are characterized by their lightweight design and high energy density, making them an attractive option for various applications.
Components of Zinc-Air Batteries
Zinc-air batteries consist of several essential components:
- Anode: Made primarily of zinc, which serves as the fuel for the battery.
- Cathode: Typically composed of a porous carbon material that allows oxygen to enter while facilitating the reduction reaction.
- Electrolyte: An alkaline solution, commonly potassium hydroxide, that conducts ions between the anode and cathode.
- Separator: A membrane that prevents direct contact between the anode and cathode while allowing ionic movement.
Part 2. How do zinc-air batteries work?
Zinc-air batteries produce electricity using a combination of zinc and oxygen from the air. The core process involves specific chemical reactions within the battery, making it a unique energy source.
1. Anode Reaction: Oxidation of Zinc
The battery contains a zinc anode made of zinc powder. The anode’s zinc undergoes an oxidation reaction when you use the battery. In this reaction, zinc loses electrons, turning into zinc ions (Zn²⁺). The chemical equation for this reaction is:
Zn → Zn²⁺ + 2e⁻
This reaction is crucial because it releases the electrons that will later flow through an external circuit, providing the electrical energy needed to power devices.
2. Cathode Reaction: Reduction of Oxygen
At the cathode, a completely different reaction takes place. Oxygen from the air enters the battery through tiny openings in the casing, reaching the cathode. Here, the oxygen undergoes a reduction reaction, meaning it gains electrons. This reaction also involves water (H₂O) from the electrolyte and can be represented by the following equation:
O₂ + 2H₂O + 4e⁻ → 4OH⁻
This reaction produces hydroxide ions (OH⁻) moving through the electrolyte towards the zinc anode.
3. Role of the Alkaline Electrolyte
The electrolyte in zinc-air batteries is typically an alkaline solution, often potassium hydroxide (KOH). This electrolyte facilitates the movement of hydroxide ions between the cathode and anode, allowing the chemical reactions to continue smoothly. The flow of these ions within the battery and the flow of electrons through the external circuit generate an electric current that powers the connected device.
4. Overall Reaction
The overall reaction inside the battery combines the anode and cathode reactions. The result is the conversion of zinc and oxygen into zinc oxide (ZnO) while producing electricity:
2Zn + O₂ + 2H₂O → 2ZnO + 4OH⁻
The efficiency of zinc-air batteries comes from their ability to use oxygen from the air. This means they don’t need to carry all the reactants internally, making them lightweight and energy-dense.
Part 3. Advantages of zinc air batteries
Zinc-air batteries offer numerous benefits, including:
- High Energy Density: They provide a higher energy density than conventional batteries, making them suitable for applications requiring long-lasting power.
- Environmentally Friendly: Zinc is abundant and non-toxic, making these batteries more ecologically friendly than lithium-ion or lead-acid batteries.
- Cost-Effective: The materials used in zinc-air batteries are relatively inexpensive, contributing to lower production costs.
- Lightweight: Their lightweight design makes them ideal for portable applications like hearing aids and electric vehicles.
Part 4. Applications of zinc air batteries
Zinc-air batteries have a wide range of applications, including:
- Hearing Aids: Their compact size and high energy density make them perfect for powering hearing aids.
- Electric Vehicles: Due to their lightweight and high energy capacity, Zinc-air batteries are being explored as a potential power source for electric vehicles.
- Renewable Energy Storage: They can store energy from renewable sources like solar and wind.
- Consumer Electronics: Devices like cameras and remote controls can benefit from the long-lasting power of zinc air batteries.
Part 5. Challenges facing zinc air batteries
Despite their advantages, zinc-air batteries face several challenges:
- Limited Rechargeability: Traditional zinc air batteries are not easily rechargeable, which limits their lifespan and usability in specific applications.
- Air Management: Maintaining proper airflow to the cathode is crucial for performance, which can be challenging in practical applications.
- Zinc Dendrite Formation: During discharge and recharge cycles, zinc can form dendrites that may short-circuit the battery.
Part 6. How do zinc-air batteries compare to other battery types?
Zinc-air batteries can be compared to other common battery types, such as lithium-ion and lead-acid batteries, based on several key features.
- Energy Density: Zinc air batteries generally have a high energy density, which means they can store more energy in a smaller volume than lead-acid batteries. However, lithium-ion batteries typically have an even higher energy density, making them the preferred choice for many modern applications.
- Cost: Zinc-air batteries are often more cost-effective than lithium-ion batteries due to the lower cost of raw materials. Lead-acid batteries are also relatively inexpensive but may not offer the same performance.
- Environmental Impact: Zinc is abundant and non-toxic, making zinc air batteries more environmentally friendly than lead-acid batteries, which contain harmful lead. Lithium-ion batteries have a moderate environmental impact due to the mining and processing of lithium.
- Weight: Zinc-air batteries are lightweight and suitable for portable applications. Lithium-ion batteries are also lightweight, while lead-acid batteries are significantly heavier.
- Rechargeability: One of the main drawbacks of zinc-air batteries is their limited rechargeability compared to lithium-ion batteries, which people can recharge hundreds of times with minimal degradation.
| Feature | Zinc Air Battery | Lithium-Ion Battery | Lead-Acid Battery |
|---|---|---|---|
| Energy Density | High | Very High | Moderate |
| Cost | Low | High | Low |
| Environmental Impact | Low | Moderate | High |
| Weight | Light | Moderate | Heavy |
| Rechargeability | Limited | Excellent | Good |
Part 7. FAQs
-
What makes zinc air batteries unique?
Zinc air batteries are unique because they use oxygen from the air as a reactant, allowing for a higher energy density and lightweight design compared to many other battery types. -
Why are zinc-air batteries not widely used in consumer electronics?
The main reason is their limited rechargeability and the challenges associated with air management, which makes them less practical for devices that require frequent recharging. -
Are zinc air batteries safe to use?
Yes, zinc-air batteries are considered safe. They do not contain toxic materials, and their chemical reactions are stable under normal operating conditions. -
How long do zinc-air batteries last?
The lifespan of zinc air batteries can vary depending on usage and storage conditions. Still, they can provide reliable power for several weeks to months in devices like hearing aids. -
What advancements are being made in zinc-air battery technology?
Research is ongoing to improve the rechargeability and efficiency of zinc air batteries, with innovations in materials and designs aimed at overcoming current limitations.
Related Tags:
More Articles

What You Need to Know About AA 3.6V Lithium Battery
Learn all about AA 3.6V lithium batteries—voltage, size, capacity, uses, and the best replacements. Discover why they’re powerful, and highly reliable.
What Are Lithium Salts and Why They Matter in Battery Electrolytes
Lithium salts in electrolytes are key to battery performance, powering everything from phones to EVs and shaping the future of clean energy.
Lithium AAA Battery Guide: Power, Performance & Chargers
Explore lithium AAA batteries—voltage, capacity, weight, top brands, and more. Learn how to choose the best battery for your device and why it really matters.
How to Calculate Watts, Volts, and Amps (With Simple Formulas and Examples)
Learn how to calculate watts, volts, and amps for lithium batteries with simple formulas and examples, ideal for EVs, solar, and energy systems.
Comprehensive Analysis of U.S. Tariffs on Chinese Lithium Batteries
U.S. tariffs on Chinese lithium batteries in 2025 impact costs, supply chains, and EV, energy storage, and electronics industries globally.