In the world of lithium batteries, the charge-discharge curve is like a key. It unlocks profound insights into battery performance and working states. By studying these curves, we can decode the secrets behind a battery’s efficiency, capacity, and lifespan.
Let’s delve into the fascinating world of lithium battery charge-discharge curves and explore what makes them so crucial.
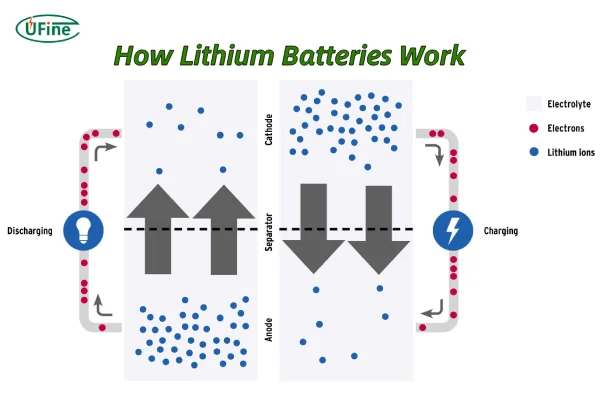
Part 1. How lithium batteries work
Firstly, we need to know how lithium batteries work. They work through a process involving the movement of lithium ions between two electrodes: the anode and the cathode.
Here’s a simplified breakdown of how they work:
Components:
- Anode: The negative electrode, typically made of graphite.
- Cathode: The positive electrode, made of a metal oxide like lithium cobalt oxide or lithium iron phosphate.
- Electrolyte: A liquid or gel-like substance that allows lithium ions to move between the electrodes.
- Separator: A porous membrane that separates the electrodes and prevents direct contact, but allows lithium ions to pass through.
Charging:
- When you connect a lithium-ion battery to a charger, an electric current is applied.
- This current causes lithium ions to move from the cathode to the anode, where they are stored within the graphite structure.
- As the lithium ions move, electrons flow through the external circuit to the anode.
Discharging:
- When you use the battery to power a device, the reverse process occurs.
- Lithium ions move from the anode to the cathode through the electrolyte.
- This movement of ions generates an electric current that powers the device.
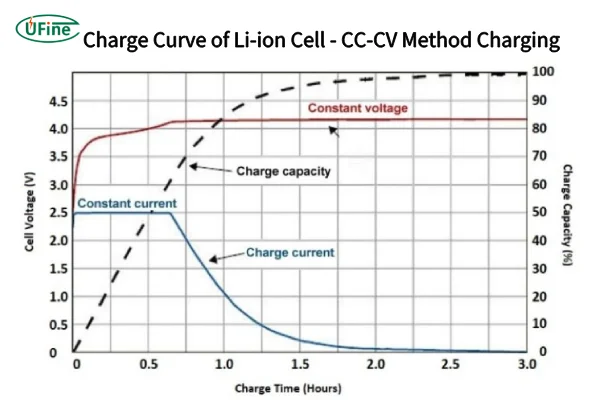
Part 2. The charging curve – energy flowing in
How to Read Lithium Battery Discharge Curve and Charging Curve?
When a lithium battery is plugged into a charger, complex chemical reactions quietly occur inside. These processes are reflected in the charging curve, which can be broken down into three phases:
1. Initial Charging Phase
At the start, the charging curve shows a steep rise in voltage. This happens because lithium ions, driven by the electric field, move quickly from the cathode (positive electrode) through the electrolyte to the anode (negative electrode).
Here, the internal resistance is low, allowing lithium ions to flow smoothly. Imagine these ions as eager residents finding new homes in the anode. The result? A rapid increase in voltage as energy is stored efficiently.
2. Middle Charging Phase
As charging progresses, the curve begins to flatten. Why? Because the cathode structure starts to change as it continues to release lithium ions. Meanwhile, the polarization effect intensifies.
Polarization occurs when resistance within the electrode and electrolyte slows the movement of ions. This reduced ion migration rate causes the voltage to increase more gradually, reflecting the increasing difficulty of storing energy in the anode.
3. Final Charging Phase
Toward the end, the curve levels off near the cutoff voltage. At this stage, the battery management system tightly regulates the charging current to prevent overcharging, which can lead to overheating, electrolyte breakdown, or structural damage to the electrodes.
By this point, most lithium ions have reached the anode, leaving only a few stragglers. Voltage growth slows to a crawl until the battery reaches full capacity, marking the end of the charging process.
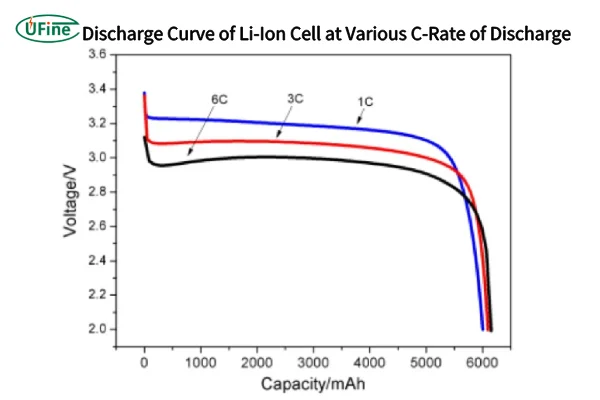
Part 3. The discharging curve – energy flowing out
When a lithium battery powers a device, its discharging curve takes over, showcasing three distinct phases as well:
1. Initial Discharge Phase
Initially, the voltage remains stable and high. Lithium ions easily leave the anode and migrate back to the cathode, creating a steady current that powers the device.
Think of this stage as a factory operating smoothly with plenty of raw materials. Energy output is consistent and efficient.
2. Middle Discharge Phase
As discharge continues, the voltage gradually drops. The structure of the anode changes as lithium ions leave, increasing internal resistance. The polarization effect grows stronger, slowing ion migration.
This phase reflects the battery working harder to sustain output, much like a factory facing logistical challenges while still managing production.
3. Final Discharge Phase
In the last stage, voltage declines rapidly. Most lithium ions have been consumed, leaving too few to maintain a stable current. Internal reactions become erratic, signaling that the battery is nearly drained.
It’s akin to a factory running out of materials, causing production to collapse. If discharge continues past this point, irreversible damage can occur, reducing battery lifespan and capacity.
Part 4. Why are charge-discharge curves important?
Analyzing charge-discharge curves offers valuable insights into battery performance. Here’s why they matter:
-
Internal Resistance The curve’s slope reveals the battery’s internal resistance. A steep rise or drop indicates high resistance, which can reduce charging efficiency and output power.
-
Capacity Evaluation The plateau length and height reflect the battery’s capacity. A longer, higher plateau means the battery can store and release more energy, which is critical for evaluating its suitability for various applications.
-
Quality Assessment Consistency in the curve across multiple cycles indicates battery quality. Fluctuations or distortions suggest chemical instability or structural damage, which can shorten lifespan.
Part 5. What affects the charge and discharge curve?
Several factors can impact a lithium battery’s charge-discharge curve, including:
1. Temperature
Low temperatures slow down chemical reactions and reduce electrolyte conductivity, increasing internal resistance. This is why cold weather can make phone batteries drain faster and take longer to charge.
2. Aging
Over time, electrode materials degrade, and electrolytes deteriorate. These changes increase resistance and reduce capacity, both of which are visible in the curve.
3. Current Levels
High currents cause stronger polarization effects, altering the curve’s slope and shortening the plateau. While fast charging saves time, it can accelerate battery wear.
Part 6. Conclusion
The charge-discharge curve serves as a vital window into a lithium battery’s health, performance, and suitability for various applications. By interpreting these curves, engineers and researchers can optimize battery design, extend lifespan, and enhance safety.
From powering electric vehicles to energizing portable devices, understanding charge-discharge curves plays a key role in advancing battery technology. As we continue to explore the intricacies of lithium batteries, we pave the way for innovations in renewable energy and sustainable technologies.
Related Tags:
More Articles

How to Choose the Best Floor Scrubber Battery for Commercial Cleaning?
Selecting the ideal floor scrubber battery ensures a long runtime, rapid charging, and minimal maintenance for efficient commercial cleaning operations.
Battery for Blower vs Battery for Leaf Vacuum: Which One Should You Choose?
Battery for blower vs leaf vacuum—learn the key differences in power, fit, and runtime to choose the right battery for your outdoor tool needs.
How to Choose the Right Battery for Blower?
Choosing the right blower battery? Consider voltage, capacity, chemistry & usage. This guide helps match the best battery for peak performance.
How to Choose the Best Insulated Battery Box for Lithium Batteries?
Choosing the Best Insulated Battery Box for Lithium Batteries? Discover key factors such as size, material, and safety for optimal protection and performance.
7 Critical Elements on a Lithium Battery Shipping Label
What must be on a lithium battery shipping label? Learn 7 key elements to ensure safety, legal compliance, and correct handling across all transport modes.