In the world of modern energy storage, lithium batteries are leading the charge. These batteries have become essential to daily life, from powering smartphones and laptops to electric vehicles and solar energy systems. But have you ever wondered what makes these batteries work so efficiently?
One of a lithium battery’s most important yet often overlooked components is the lithium salt used in the electrolyte. This article explores what lithium salts are, why they are critical to battery performance, and how they influence the future of clean energy.
Part 1. What are lithium salts?
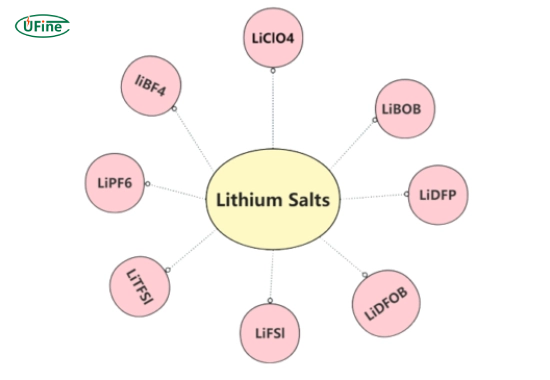
Lithium salts are chemical compounds that contain lithium ions (Li⁺) combined with other elements. These salts are typically white, crystalline powders that dissolve in solvents to form electrolytes used in lithium-ion batteries.
The most common lithium salts include:
- Lithium hexafluorophosphate (LiPF₆)
- Lithium bis(trifluoromethane sulfonyl)imide (LiTFSI)
- Lithium tetrafluoroborate (LiBF₄)
- Lithium perchlorate (LiClO₄)
These salts are unlike the table salt (sodium chloride) you use in food. Instead, they are carefully engineered substances that play a vital role in battery chemistry.
Part 2. Is lithium a salt?
Yes and no — lithium is not a salt, but it forms salts when reacting with other elements. Lithium is a soft, silver-white metal belonging to the periodic table’s alkali metal group. When lithium forms a compound with a non-metal or a negatively charged ion, it becomes a lithium salt.
So, when people ask, “Is lithium a salt?” the precise answer is:
Lithium is a metal that forms salts when combined with other chemical compounds, especially in battery electrolytes.
Part 3. What is the role of lithium salts in batteries?
In a lithium-ion battery, energy is stored and released through lithium ions flow between the anode and cathode. For this movement to happen efficiently, an electrolyte is needed.
That’s where lithium salts come in.
They create the ionic conductivity required for lithium ions to travel through the electrolyte. The battery wouldn’t charge or discharge appropriately without the right lithium salt.
The primary roles of lithium salts in batteries are:
- Conduct lithium ions between electrodes.
- Maintain thermal and chemical stability.
- Prevent side reactions by forming a solid electrolyte interface (SEI) layer.
- Ensure long battery life and consistent performance.
Artikel Terkait: What Is the Electrolyte in a Battery Made Of?
Part 4. Why is lithium hexafluorophosphate (LiPF₆) commonly used?
Among all lithium salts, LiPF₆ is the most widely used, especially in commercial lithium-ion batteries. But why?
Here’s what makes LiPF₆ the go-to choice:
- High ionic conductivity: Lithium ions move quickly in the electrolyte.
- Stable SEI layer: Helps protect the anode and extend battery life.
- Good solubility: Dissolves well in organic solvents like ethylene carbonate (EC) and dimethyl carbonate (DMC).
- Acceptable thermal stability: Works safely under normal operating conditions.
However, LiPF₆ is not perfect. It can degrade at high temperatures or when exposed to moisture, releasing harmful gases like hydrofluoric acid (HF). That’s why researchers are exploring alternatives.
Part 5. What are the alternatives to LiPF₆?
Scientists are developing safer and more stable lithium salts as battery technology evolves. Some of the most promising alternatives include:
LiTFSI (Lithium bis(trifluoromethane sulfonyl)imide)
- Excellent thermal stability
- High ionic conductivity
- More expensive and slightly more corrosive
LiFSI (Lithium bis(fluoro sulfonyl)imide)
- Superior thermal and chemical stability
- Better performance in high-voltage batteries
LiBF₄ (Lithium tetrafluoroborate)
- More moisture-resistant than LiPF₆
- Slightly lower conductivity
These alternatives are being tested in next-generation batteries, especially for electric vehicles (EVs) and grid energy storage.
Part 6. How do lithium salts affect battery performance?
The type and concentration of lithium salt used in the electrolyte greatly affect a battery’s:
- Cycle life (how many times it can be charged and discharged)
- Charging speed
- Operating temperature range
- Safety and stability
- Energy density
For example, a poorly chosen salt might lead to dendrite formation, which can cause short circuits and even explosions. On the other hand, a good lithium salt ensures smooth ion flow and stable chemical reactions, making the battery more reliable and longer-lasting.
Part 7. Are lithium salts safe?
Safety is always a top concern with lithium-ion batteries. While lithium salts like LiPF₆ are generally safe under normal conditions, they can become hazardous if:
- Exposed to high temperatures
- In contact with moisture
- Damaged or leak
Some lithium salts, especially LiPF₆, can release toxic or corrosive gases under stress. That’s why battery packs often include:
- Thermal management systems
- Moisture-proof seals
- Battery management software
Researchers are working hard to develop non-toxic, non-flammable lithium salts to make future batteries safer.
Part 8. What is the future of lithium salts in energy storage?
The battery industry is evolving fast. As demand grows for electric vehicles, portable devices, and renewable energy storage, the need for better lithium salts becomes critical.
Future goals include:
- Higher energy density: More power in smaller batteries.
- Faster charging: Without overheating or wear.
- Longer lifespan: Up to thousands of cycles.
- Lower cost: Especially for EV applications.
- Eco-friendly production: Less environmental impact.
Next-gen lithium salts are already being used in solid-state, lithium-sulfur, and lithium-air batteries—technologies that could redefine how we store and use energy.
Part 9. How are lithium salts manufactured?
Producing lithium salts involves chemical synthesis and purification steps. Here’s a simplified version of the process:
- Extract lithium from brine or hard rock.
- React the lithium with acids or fluorine compounds to produce salts.
- Dry and purify the product to remove moisture and impurities.
- Pack the salts in moisture-resistant containers for battery use.
Because of the complexity and cost, only a few manufacturers specialize in high-purity lithium salts suitable for batteries.
Part 10. What are the environmental concerns?
While lithium salts make clean energy possible, there are environmental challenges, such as:
- Mining impact: Extracting lithium can use a lot of water and harm ecosystems.
- Chemical waste: Toxic byproducts from salt production.
- Battery disposal: Improper disposal can release harmful chemicals.
To reduce the impact, companies are:
- Recycling lithium batteries
- Using greener solvents
- Improving salt production efficiency
In the future, closed-loop systems may allow lithium salts to be recovered and reused from old batteries.
Part 11. FAQs about lithium salts
What is a lithium salt used for?
Lithium salts are primarily used in battery electrolytes to conduct lithium ions between electrodes, enabling the battery to charge and discharge.
Is lithium a salt or a metal?
Lithium is a metal that forms salts when it bonds with other elements, such as fluorine or boron, in battery applications.
Why is LiPF₆ used in lithium-ion batteries?
LiPF₆ offers high ionic conductivity, stability, and good solubility in organic solvents, making it ideal for most commercial lithium-ion batteries.
Are lithium salts flammable?
The salts are not always flammable, but when mixed with organic solvents, the resulting electrolyte can be combustible, especially under heat or stress.
Can lithium salts be recycled?
Recycling efforts are underway to recover lithium salts from old batteries, helping reduce environmental impact and resource consumption.
Related Tags:
More Articles

What You Need to Know About AA 3.6V Lithium Battery
Learn all about AA 3.6V lithium batteries—voltage, size, capacity, uses, and the best replacements. Discover why they’re powerful, and highly reliable.
Lithium AAA Battery Guide: Power, Performance & Chargers
Explore lithium AAA batteries—voltage, capacity, weight, top brands, and more. Learn how to choose the best battery for your device and why it really matters.
How to Calculate Watts, Volts, and Amps (With Simple Formulas and Examples)
Learn how to calculate watts, volts, and amps for lithium batteries with simple formulas and examples, ideal for EVs, solar, and energy systems.
Comprehensive Analysis of U.S. Tariffs on Chinese Lithium Batteries
U.S. tariffs on Chinese lithium batteries in 2025 impact costs, supply chains, and EV, energy storage, and electronics industries globally.
What Are Lithium Button Batteries?
Learn about lithium button batteries (CR2032, CR2025), their uses, safety tips, and how to replace them properly.