The lithium batteries of many digital devices are affected by low temperatures. So how does low temperature affect the performance of lithium batteries? How to reduce the impact of low temperature on lithium batteries?
Part 1. What effect does low temperature have on lithium batteries?
1. Charging and discharging lithium-ion batteries at low temperatures
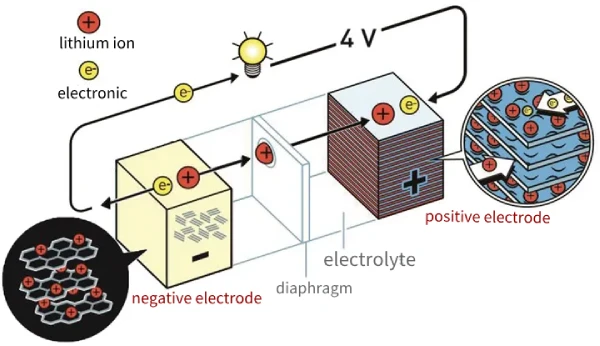
The positive electrodes of lithium-ion batteries are generally NCM, LFP, and LCO, and the negative electrodes are graphite (Gr).
During charging, lithium ions come out of the positive electrode lattice, pass through the electrolyte separator, and go to the negative electrode, where they are embedded between the graphite layers. It can be understood when discharging as coming out of the graphite negative electrode layer and then returning to the positive electrode lattice.
When the temperature is low, the mobility of molecules decreases. The entire reaction speed and material transfer process will slow down. Then, the most obvious thing that is slow in the battery is the transport of lithium ions/lithium atoms between graphite (negative electrode) layers and in the positive electrode lattice. As a result, a large amount of lithium accumulates at the interface between the electrode and the electrolyte.
When charging, if lithium ions cannot squeeze into the graphite layer, they will directly obtain electrons on the surface of the negative electrode and turn into metallic lithium, accumulating into lithium dendrites.
During discharge, lithium ions are squeezed on the surface of the cathode lattice, which can easily cause the cathode to rupture.
2. Low-temperature charging leads to lithium precipitation, causing safety hazards
Lithium ions enter the graphite layers in an orderly manner when a normal battery is charged, and an intercalation reaction occurs. However, when charging at low temperatures, lithium ions cannot squeeze into the graphite layer. They will directly obtain electrons on the surface of the negative electrode and turn into metallic lithium, which becomes a conversion reaction (the reaction potential is lower than the intercalation reaction, which can be understood as more difficult to occur, but the intercalation reaction It is difficult for the substance to diffuse, making the conversion reaction easy to occur at low temperatures), and accumulates into lithium dendrites.
Metal lithium is very active and can react immediately with the electrolyte. The generated material is also irreversible, causing capacity loss.
In addition, the continued growth of metallic lithium can easily pierce the separator and connect to the positive electrode, causing an internal short circuit and easily causing serious safety accidents.
3. Low-temperature discharge induces rupture of cathode particles
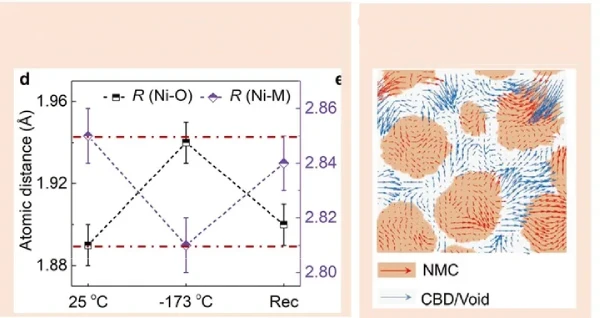
During low-temperature discharge, lithium ions are squeezed on the surface of the cathode lattice, which can easily cause the rupture of the cathode active particles. On the one hand, it leads to the loss of positive active materials and capacity loss. In addition, the precipitation of transition metal elements in the positive electrode will migrate to the surface of the negative electrode and transform into metal particles, inducing lithium deposition.
4. Low-temperature storage reduces battery capacity
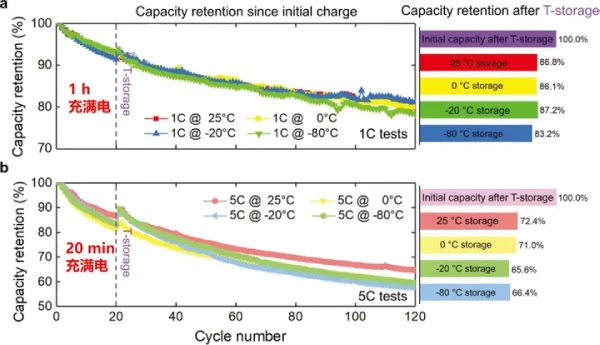
Recently, researchers discovered that the battery was only left at low temperature for 48 hours, then left at room temperature, and then capacity tested. It was found that a battery that has been left standing at low temperature for a long time will lose 3.2% of its capacity when charged and discharged at a small rate (slow charge). However, when charging and discharging at high rates (fast charging), the capacity loss reaches 6%.
In summary, the reason for the capacity loss caused by being placed at a low temperature is that the low temperature aggravates the rupture of the cathode. On the other hand, low temperature may cause the positive electrode particles to rotate, causing them to separate from the binder and lose electrochemical activity.
Part 2. Why does low temperature affect lithium-ion battery performance?
As mentioned above, lithium batteries’ working (discharging) principle is that the lithium ions in the negative electrode are dissociated through the electrolyte, pass through the battery separator, and move back to the positive electrode to generate current. The higher the discharge capacity, the more lithium ions that return to the positive electrode.
In low-temperature environments, the chemical substances inside the battery react slowly. Relatively speaking, as lithium ions’ mobility worsens, it becomes more difficult for lithium ions to pass through the separator, and the energy charge and discharge efficiency naturally decrease.
In extreme low-temperature conditions, the electrolyte may freeze, and the battery cannot be discharged, seriously affecting the low-temperature performance of the battery system.
Some data show that the internal resistance of lithium-ion batteries increases at low temperatures, the activity decreases, and the charge and discharge power decreases significantly. At -20℃, the power battery can only output about 60% of the power. Moreover, low-temperature charging is prone to lithium precipitation in the negative electrode, seriously affecting battery life and safety.
Part 3. How to use lithium batteries at low temperatures?
1. Use batteries suitable for low-temperature environments
Choose a battery type with low-temperature characteristics. Examples include low-temperature lithium-ion batteries, nickel-metal hydride batteries, or sodium-sulfur batteries. These batteries are specifically designed to provide better performance at low temperatures.
2. Maintain proper temperature control
When using the battery in a low-temperature environment, try to keep the battery within the appropriate temperature range. Insulating materials or insulated packaging can reduce temperature exchange between the battery and the external environment. In addition, consider using battery heating devices or heating systems to provide a suitable warm environment.
3. Warm up the battery
Place the battery in a warmer environment to warm it up before use to improve its performance. You can use specialized battery heating equipment or leave the battery in a warm room for some time.
4. Optimize battery management system
The battery management system can be optimized to adapt to low-temperature conditions for applications that require long-term use of batteries in low-temperature environments. Maximize battery performance and life by adjusting charge and discharge strategies and optimizing battery temperature control and protection mechanisms.
5. Regular inspection and maintenance
Regularly check batteries used in low-temperature environments to ensure they are functioning properly. Keep the battery clean and avoid water or ice on the battery surface. Per the manufacturer’s recommendations, perform battery maintenance and care.
6. Consider backup power
In extreme cold conditions, battery performance can be severely affected. Therefore, in some critical applications, backup power supplies or redundant systems can be considered to ensure continuity of energy supply. This could be other types of batteries, fuel cells, or other energy storage devices.
Part 4. Summary
The above are some common methods and suggestions to help better solve the problem of using batteries at low temperatures. In each case, choose the appropriate solution based on the application requirements and the battery manufacturer’s guidance.
Related Tags:
More Articles
Tracker Lithium Batteries: A Comprehensive Guide
Learn about tracker lithium battery types, applications, advantages, and how to choose the right manufacturer for GPS and IoT tracking devices.
8 Volt Golf Cart Batteries: Tips, Types & Lifespan
Explore 8 volt golf cart batteries, types, lifespan, and maintenance tips. Find out which battery suits your cart best in 2026.
What Is the Charge Voltage of the AGM Battery?
Discover the ideal charge voltage for AGM batteries. Ensure optimal performance and longevity. Learn more about maintaining your battery today!
Bad Battery Cell Symptoms: How to Tell If a Battery Cell Is Dead
Learn how bad battery cells behave, how to test them, and when replacement is the safest option.
18650 Maximum Capacity in 2026: What’s Real and What’s Hype?
Discover the real 18650 battery capacity limits in 2026, top high-capacity cells, and how to avoid fake claims.