Batteries have become an essential part of modern life. From powering our smartphones to our electric vehicles, we rely on them daily. But have you ever wondered about the different types of batteries available and how they work? One fascinating battery technology is the oxide battery. While not as widely known as lithium-ion or alkaline batteries, oxide batteries hold great potential for various applications.
In this article, we will explore oxide batteries, their functions, and their differences from other types of batteries. You’re in the right place if you’re curious about their unique advantages and limitations. Let’s explore this battery technology and understand its potential!
Part 1. What is an oxide battery?
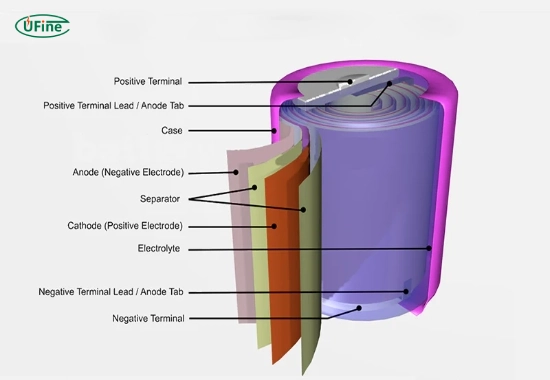
An oxide battery is a type of battery that uses metal oxides as one of its key components in the electrochemical reaction that generates electricity. The basic principle behind all batteries is converting chemical energy into electrical energy, and oxide batteries do this through a specific chemical reaction involving metal oxides.
Key components of an oxide battery
The key components of an oxide battery include:
- Anode: Typically made of a metal, the anode undergoes oxidation during discharge, releasing electrons.
- Cathode: The cathode is composed of a metal oxide material. During discharge, it absorbs electrons, creating a flow of electric current.
- Electrolyte: This medium facilitates the flow of ions between the anode and cathode.
- Separator: Keeps the anode and cathode from directly touching while allowing the flow of ions.
Part 2. How does an oxide battery work?
The basic working principle of an oxide battery revolves around redox reactions—the process where the anode material loses electrons (oxidation) and the cathode material gains electrons (reduction). When the battery discharges, electrons flow from the anode through an external circuit to the cathode, providing electrical energy to power devices.
The chemical reaction in oxide batteries
In a typical oxide battery, the cathode’s metal oxide (such as iron oxide or copper oxide) plays a critical role. During discharge, the metal oxide accepts electrons, causing a reduction reaction. This makes the metal oxide more electrically conductive and allows for energy release.
Part 3. Types of oxide batteries
1. Iron oxide batteries
Iron oxide batteries use iron oxide (Fe2O3) as the cathode. They’re cost-effective and suitable for large-scale energy storage. Their low cost and sustainability make them attractive for applications like grid storage.
- Pros: Cheap materials. Environmentally friendly.
- Cons: Lower energy density than lithium-ion.
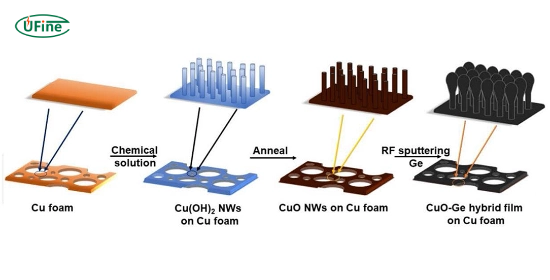
Copper oxide batteries use copper oxide (CuO) as the cathode. They perform well in high-power applications and have better conductivity than iron oxide batteries.
- Pros: Efficient in high-power uses. Stable in cooler environments.
- Cons: Lower energy density than lithium-ion.
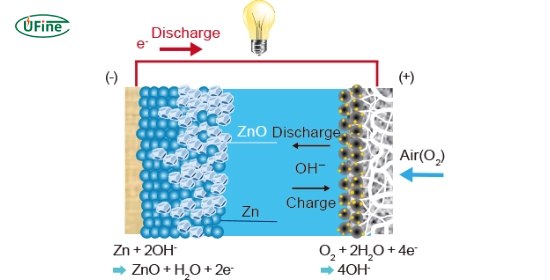
3. Zinc oxide batteries
Zinc oxide batteries are known for their high energy density. This makes them promising for electric vehicles and large-scale storage.
- Pros: High energy density. Suitable for significant storage needs.
- Cons: It is more complex to manufacture.
Part 4. Oxide battery vs. lithium-ion battery
Energy Density
Lithium-ion batteries are smaller and more powerful. They can store more energy in less space, making them ideal for electronics and electric vehicles. Oxide batteries generally have lower energy density, so they’re not as compact or powerful.
Cost and Sustainability
Oxide batteries use cheaper materials (like iron and copper), making them more affordable and sustainable than lithium-ion batteries, which rely on expensive and environmentally damaging materials like lithium and cobalt.
Charging Speed
Lithium-ion batteries charge faster and have higher discharge rates, so they’re preferred for high-demand devices. Oxide batteries are slower to charge and discharge, limiting their use in power-hungry applications.
Part 5. Oxide battery vs. alkaline battery
Energy Output
Alkaline batteries are great for low-power devices like remotes. They provide a steady power supply, but only for small devices. Oxide batteries are more efficient and better for high-power systems, such as industrial machinery or backup power systems.
Cost
Alkaline batteries are cheaper, but oxide batteries last longer and are more cost-effective in high-demand applications.
Environmental Impact
Oxide batteries are generally greener. Alkaline batteries contain harmful metals, whereas oxide batteries use more recyclable materials.
Part 6. Advantages of oxide batteries
While oxide batteries are still evolving, they offer several advantages over other types of batteries:
- Cost-effective: Due to the availability of metal oxides, oxide batteries can be produced at a lower cost.
- Longer lifespan: Oxide batteries often last longer than traditional batteries, especially in low-energy devices.
- Environmentally friendly: Metal oxides are generally less harmful to the environment than the materials used in lithium-ion or alkaline batteries.
- Better performance in specific applications: Oxide batteries can perform better than alkaline batteries in applications requiring high power output.
Part 7. Disadvantages of oxide batteries
Despite their advantages, oxide batteries have some limitations that may hinder their widespread adoption:
- Lower energy density: Oxide batteries generally offer lower energy density than lithium-ion or other advanced batteries.
- Limited applications: Due to their lower power output, oxide batteries may not be suitable for high-demand applications like smartphones or electric vehicles.
- Longer charging times: Some oxide batteries may take longer to charge than others.
Part 8. The future of oxide batteries
Researchers are actively exploring ways to improve the performance of oxide batteries. With the growing demand for sustainable energy solutions, oxide batteries may play a critical role in the future of energy storage.
Advancements in oxide battery technology
- Improved energy density: Scientists are increasing the energy density of oxide batteries to make them more competitive with lithium-ion batteries.
- Better materials: Researchers are exploring new types of metal oxides that could enhance the performance of oxide batteries.
- Hybrid technologies: The combination of oxide and other battery technologies may create new opportunities for energy storage solutions.
Part 9. Applications of oxide batteries
Oxide batteries have several potential applications, including:
- Energy storage: They can be used for large-scale energy storage systems to support renewable energy.
- Electric vehicles: As their energy density improves, oxide batteries may become a cost-effective solution for electric cars.
- Consumer electronics: While not widely used, oxide batteries could find their place in low-power electronic devices.
- Military and aerospace: Oxide batteries could be used in specialized applications due to their stability and durability.
Part 10. FAQs
-
What is the main advantage of an oxide battery?
Oxide batteries, which use abundant metal oxides, have the main advantages of being cost-effective and potentially achieving environmental sustainability. -
Are oxide batteries better than lithium-ion batteries?
While oxide batteries are cost-effective and environmentally friendly, lithium-ion batteries generally offer better energy density and longevity performance. -
Can oxide batteries be used in electric vehicles?
Although oxide batteries currently have a lower energy density, advancements in technology could make them viable for electric vehicle applications in the future. -
How long do oxide batteries last?
Oxide batteries have a longer lifespan than alkaline batteries. Still, they may not last as long as lithium-ion batteries in high-demand applications. -
Are oxide batteries environmentally friendly?
Yes, oxide batteries are more environmentally friendly compared to lithium-ion batteries, as they rely on metal oxides instead of materials like cobalt and lithium.
Related Tags:
More Articles

The Ultimate Guide to 12V 18650 Battery Packs: Design, Benefits, and Applications
Looking for a reliable 12V power source? This guide covers 12V 18650 battery packs, their design, benefits, and applications.
9 Volt Battery Capacity Showdown: Lithium vs. Alkaline Batteries
Maximizing 9V battery capacity? This in-depth guide will help you compare lithium vs. alkaline batteries to choose the best option for your needs.
2200mAh Battery: Will It Meet Your Power Needs?
Is a 2200mAh battery right for you? Consider battery life, device usage, and cost. Discover if it meets your power needs. Read our guide now!
How to Pick the Best 12V 4Ah Battery?
Need a 12V 4Ah battery? We'll help you choose! Find compatible options for your electronics or mobility scooter. Read our simple tips and buy confidently!
Deep Cycle Camper Battery: Is It Right for You?
Deep cycle camper batteries boost power. They're reliable and last. Learn if this upgrade's worth it for your RV adventures. Read now!