- Part 1. What is a battery electrolyte?
- Part 2. Key components of battery electrolytes
- Part 3. Why is the electrolyte important in a battery?
- Part 4. What do manufacturers use in battery electrolytes?
- Part 5. How do electrolytes affect battery performance?
- Part 6. What are the challenges of battery electrolytes?
- Part 7. What are researchers making advancements in battery electrolytes?
- Part 8. Can battery electrolytes be recycled?
- Part 9. How do solid-state electrolytes improve battery safety?
- Part 10. FAQs
Batteries power our modern world, from smartphones to electric cars. But have you ever stopped to think about what makes them work? One critical part of any battery is its electrolyte. Without it, no battery could store or release energy. So, what is the electrolyte in a battery made of, and why is it so important? This article will briefly break it down while diving deeper into the science behind electrolytes. Read more about battery electrolytes’ materials, roles, and challenges.
Part 1. What is a battery electrolyte?
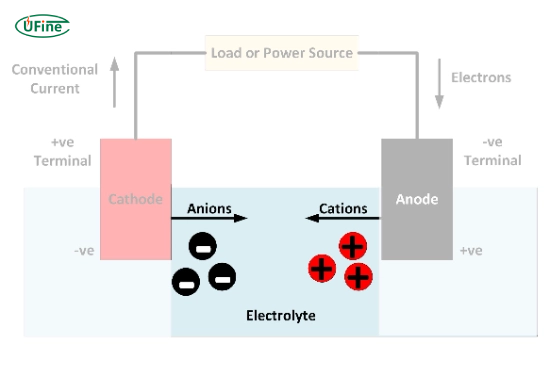
An electrolyte is a substance that allows ions to move between a battery’s positive and negative sides. This movement of ions powers devices, and batteries wouldn’t work without electrolytes.
Electrolytes can be liquid, gel, or solid. Their main job is to conduct ions while keeping electrons from flowing freely. This separation is key to producing electricity safely and efficiently.
In short, the electrolyte is like the bridge in a battery. It allows chemical reactions, which is how energy is stored and released.
Part 2. Key components of battery electrolytes
Now that we understand the basic types of electrolytes let’s examine the key components of a battery electrolyte. Manufacturers choose these components for their ability to conduct ions efficiently and compatibility with the battery’s chemistry.
1. Solvents
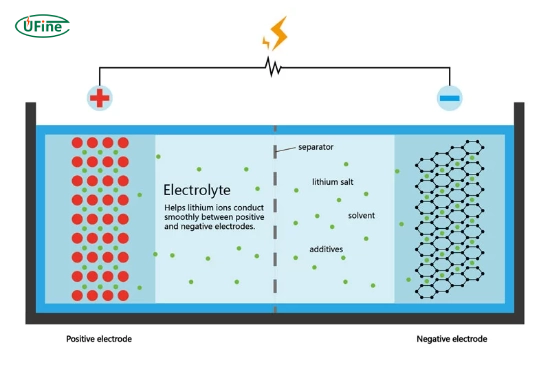
Solvents dissolve the electrolyte salts in liquid electrolytes and help ions move between electrodes. Common solvents include carbonates, such as ethylene carbonate and dimethyl carbonate, used in lithium-ion and nickel-metal hydride (NiMH) batteries. These solvents provide a medium for the movement of lithium ions, crucial for energy storage and transfer.
2. Salts
Salts are the source of ions in the electrolyte. Lithium salts like LiPF6 (Hexafluorophosphate) are commonly used in lithium-ion batteries. These salts dissociate into positively charged lithium ions and negatively charged anions, enabling the flow of electricity when the battery is in use. For sodium-ion batteries, sodium salts such as NaPF6 serve the same purpose.
3. Additives
Additives are chemicals introduced into the electrolyte to improve the battery’s performance, lifespan, or safety. These can include stabilizers to prevent degradation, flame retardants to reduce the risk of fires, and conductivity enhancers to improve ion flow.
Part 3. Why is the electrolyte important in a battery?
The electrolyte is the heart of a battery’s chemical reaction. Here’s why it’s so essential:
Ion transfer allows ions to move between the battery’s positive and negative sides, creating electricity.
- Energy storage: Without an electrolyte, a battery couldn’t store energy for later use.
- Safety: A well-designed electrolyte ensures stable performance and reduces the risk of overheating or leaks.
For example, in lithium-ion batteries, the electrolyte helps lithium ions move back and forth during charging and discharging. This movement powers everything from laptops to electric cars. These devices wouldn’t work efficiently—or safely without a stable electrolyte.
Part 4. What do manufacturers use in battery electrolytes?
The materials in an electrolyte depend on the type of battery. Below are some common examples:
1. Lead-acid battery electrolytes
- Material: Diluted sulfuric acid.
- Role: Conducts ions to generate electricity.
- Use: Found in car batteries and backup power systems.
2. Lithium-ion battery electrolytes
- Material: Organic solvents mixed with lithium salts (e.g., hexafluorophosphate).
- Role: Supports lithium-ion movement for charging and discharging.
- Use: Smartphones, laptops, and electric vehicles.
Where Do Lithium Batteries Come From?
3. Alkaline battery electrolytes
- Material: Potassium hydroxide solution.
- Role: Enables efficient ion transfer.
- Use: Everyday devices like remotes and flashlights.
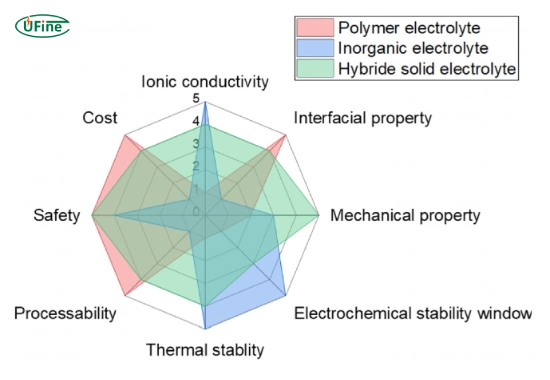
4. Solid-state battery electrolytes
- Material: Ceramics or polymers.
- Role: Provides a safer and more durable alternative to liquid electrolytes.
- Use: Emerging in electric vehicles and next-gen electronics.
5. Gel electrolytes
- Material: Liquid electrolytes turned into gel by adding silica.
- Role: Reduces the risk of leaks while maintaining performance.
- Use: Found in gel lead-acid batteries for safer applications.
Different types of batteries use different electrolytes, each optimized for specific uses and performance.
Part 5. How do electrolytes affect battery performance?
The type and quality of an electrolyte can significantly impact how well a battery performs. Here’s how:
- Energy capacity: A good electrolyte ensures a battery can store more energy. Lithium-ion batteries, for example, have high energy density thanks to their advanced electrolytes.
- Lifespan: Stable electrolytes reduce wear and tear inside the battery, making it last longer.
- Safety: Solid or gel electrolytes minimize risks like leaks or overheating, making batteries safer.
- Efficiency: High-quality electrolytes enable faster charging and discharging without losing energy.
For instance, the liquid electrolyte in a lead-acid battery is stable and reliable. Still, it’s heavy and less efficient than the electrolytes in lithium-ion batteries.
Part 6. What are the challenges of battery electrolytes?
While electrolytes are crucial to battery function, they come with their own set of challenges:
1. Safety issues
Liquid electrolytes in lithium-ion batteries are flammable and can cause fires if mishandled.
2. Degradation
Over time, electrolytes can break down, reducing battery performance. This is why batteries lose capacity as they age.
3. Environmental impact
Some electrolytes, like sulfuric acid, harm the environment if not disposed of properly.
4. Cost
High-performance electrolytes, such as those in solid-state batteries, are expensive.
Researchers are working on new materials and designs to address these issues. For example, solid-state batteries aim to eliminate the safety risks of liquid electrolytes.
Part 7. What are researchers making advancements in battery electrolytes?
The demand for better batteries has led to exciting advancements in electrolyte technology:
- Solid-state electrolytes: Developers create these for electric vehicles and other high-performance applications, offering higher safety and energy density.
- Eco-friendly electrolytes: Scientists are exploring bio-based and water-based electrolytes to reduce environmental harm.
- Ionic liquids: These are non-flammable and stable, making them a promising alternative to traditional liquid electrolytes.
- Hybrid systems: Combining solid and liquid electrolytes could offer the best of both worlds.
These innovations aim to create batteries that are safer, more efficient, and better for the planet.
Part 8. Can battery electrolytes be recycled?
Yes, battery electrolytes can be recycled, but it depends on the type of battery:
- Lead-acid batteries: These are highly recyclable, including their sulfuric acid electrolyte.
- Lithium-ion batteries: Recycling is more complex due to the various chemicals involved, but progress is being made.
- Alkaline batteries: Recycling is possible but less common due to their lower environmental impact.
Recycling electrolytes reduces waste and helps recover valuable materials, making them essential to sustainable energy systems.
Part 9. How do solid-state electrolytes improve battery safety?
Solid-state electrolytes are a game-changer for battery safety. Unlike liquid electrolytes, they don’t leak or catch fire. They are also more durable and can handle higher energy densities, making them ideal for electric vehicles and other demanding applications.
However, solid-state batteries are still expensive and not widely available. As manufacturing improves, these safer batteries are expected to become more common.
Part 10. FAQs
-
What is the electrolyte in a battery?
The electrolyte is the material that allows ions to flow between the battery’s positive and negative sides. It can be a liquid, gel, or solid. -
Why is the electrolyte important in a battery?
The electrolyte enables the chemical reactions that store and release energy. Without it, no battery could function. -
Are solid-state electrolytes better than liquid ones?
Yes, solid-state electrolytes are safer and more durable, but they are still under development and expensive to produce. -
Can battery electrolytes be reused?
Yes, electrolytes in some batteries, like lead-acid ones, can be recycled. However, recycling lithium-ion electrolytes is more challenging. -
What happens if a battery’s electrolyte leaks?
An electrolyte leak can damage the battery and pose safety risks, such as fire or chemical burns.
Related Tags:
More Articles
What is the Difference Between Silver Zinc Battery vs. Lithium-ion Rechargeable?
Compare silver zinc and lithium-ion rechargeable batteries: energy density, cycle life, safety, cost, and uses in drones, medical devices, EVs, and electronics.
What are Watts and Watt Hours in Battery?
Understand watt vs watt-hour in batteries: key differences, how to calculate capacity, and why they matter. Includes free comparison table.
Best 10 Blood Pressure Monitor Battery Review: Finding the Most Reliable
Are you looking for a reliable Blood Pressure Monitor battery? Here is a complete guide with the top 10 best blood pressure monitor batteries.
Bluetooth Headphone Battery Guide: All You Need to Know
Maximize headphone battery life with expert tips! Learn how to charge, check, troubleshoot, and choose the best bluetooth headphone battery in 2025.
LiFePO4 Battery VS. Lithium-ion Polymer Battery: Which One Is Best?
Comprehensive comparison of LiFePO4 vs Lithium Ion Polymer batteries: energy density, safety, lifespan, cost. Find out which battery suits your needs in 2025.