Lithium batteries are powering every device in today’s world, but have you ever tried to know how lithium batteries are made? Knowing the raw material used and the process of making lithium batteries can help you better understand the lithium battery working mechanism. This article will explore how lithium batteries are made, from raw materials to manufacturing and assembling processes.

Part 1. How to make lithium batteries?
The battery-making process is divided into different steps to understand better how lithium batteries are made. A lithium battery passes through different assembly lines until the final testing. Here are some important steps in making lithium batteries.
Step 1. Making Electrode
The process involves mixing electrode materials with a conductive binder to create a uniform slurry with a solvent. The anode is Carbon, and the cathode is Lithium metal oxide. The slurry is then coated on both sides of the current collector using an application tool like a slot die, doctor blade, or anilox roller. The thickness of the electrode coating can be controlled in the coating machine. This process involves vacuum mixers and coating machines.
Step 2. Drying and Cutting
In this step, the manufacturer dries the coated foil using large ovens to evaporate the solvents. After drying, the electrode sheets are cut according to their precise shape and size. They utilize slitting machines for accurate electrode cutting.
Step 3. Stacking and Filling
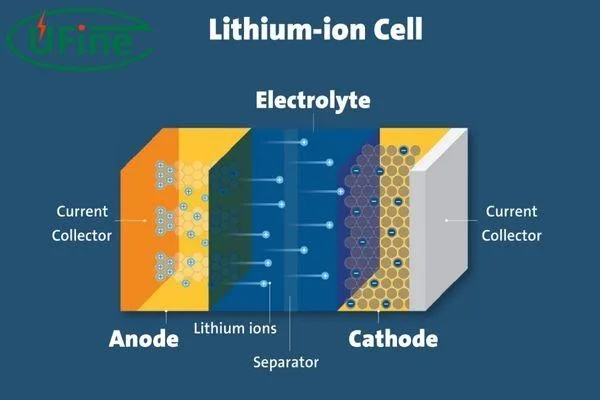
The next step is stacking each battery component in the battery casing. It can be any shape, including cylindrical, prismatic, button, or other. They place separators in between positive and negative electrodes. It helps to create a safety barrier between the positive and negative battery parts. Afterward, they fill electrolytes inside the battery for easy lithium ion movement from the cathode to the anode during the charging and discharging process.
Step 4. Sealing and Formation
It is important to know how lithium batteries are made, as battery sealing is done using heat sealers or laser welding machines. Sealing the battery does not let the electrolytes leak of the battery. Once the battery passes the sealing process, it goes to the formation process. The battery undergoes initial charging and discharging procedures to form and establish a stable solid electrolyte interphase ( SEI). This process activates the battery by ensuring it is properly working.
Step 5. Final Testing
In the final step, the battery undergoes a rigorous testing procedure. The manufacturer tests the battery capacity, charging speed, discharging rate, battery temperature, and peak load performance tests. From making electrodes to final testing, you have understood how lithium batteries are made well.
Part 2. What raw materials are needed to make lithium batteries?
A lithium battery is a combination of several materials in a unique form. Each material plays its role in delivering high power and a long life span. We will discuss all the materials one by one to sort out how lithium batteries are made.
1. Cathode Material
그리고 cathode is a positive electrode of the battery. The raw material for making cathode can vary from one battery to another battery type. For making cathode, manufacturers use lithium cobalt oxide (LiCoO2), lithium iron phosphate (LiFePO4), or nickel-manganese-cobalt oxide (NMC), depending on the battery type. The cathode absorbs hydroxide during charging and releases it during discharge.
2. Anode Material
The anode is the negative part of the battery made of graphite and, in some cases, silicon material. The anode releases electrons through oxidative reactions. Li-ion batteries typically use graphite, a carbon (C) material, with its layered structure allowing lithium ions to enter and exit during charging and discharge. Moreover, we can use lithium material as an anode, but it has some challenges. It also costs more than graphite, which is cheap and easily available.
3. Separator Material
The separator is an important element in a battery that works as a safety barrier between positive and negative parts. Manufacturers use polyolefin-based materials such as polyethylene, polypropylene, and PVC. They also use their blends of polyethylene and polypropylene, which are widely popular.
4. Electrolyte Material
Electrolytes work as a medium for the smooth flow of lithium ions from the cathode to the anode and anode to the cathode. The purpose of electrolytes is to cause less resistance so the battery can work for a long time. It uses lithium salt dissolved in an organic solvent, which does not cost much.
Part 3. How to make Lithium cells?
Making lithium cells also follows the same method we discussed above. How are lithium batteries made? The only difference is the size and shape of the both.
However, here is the lithium cell manufacturing process.
- Mix electrode materials with conductive binders to create a uniform slurry.
- Coat the slurry on the current collector using tools like a slot die, doctor blade, or anilox roller.
- The large ovens dry the coated foil to evaporate solvents.
- Cut the electrode sheets according to the precise shape and size.
- Each battery component is stacked in the battery casing.
- Place a separator between positive and negative electrodes.
- Machines inject the electrolytes inside the battery for easy lithium ion movement.
- Seal the battery using heat sealers or laser welding machines.
- Final testing of the battery to verify different metrics.
Part 4. How to assemble lithium battery packs?
Now, it’s time to assemble lithium battery packs after manufacturing small lithium cells. The small cells in the series form to increase total battery voltage and capacity. It is a useful method to make bigger lithium batteries with higher capacities. Let’s check out each step of how lithium batteries are made.
Cell Selection
Cell selection is very important for lithium battery pack cell selection. Each lithium cell should have the same capacity, chemistry, and voltage as there can be Lithium-ion (Li-ion), Lithium polymer (LiPo), Lithium iron phosphate (LiFePO4), and Nickel-metal hydride (NiMH) cells. Therefore, it is important to use a multimeter for each cell and read its brand name and description. Moreover, it is necessary to calculate the total voltage and capacity of the battery when selecting lithium cells.
구성
There are two ways to connect the cells. Either in series ( each cell positive to the other’s negative) or parallel ( each cell positive with positive and negative with negative). Connecting in series will increase overall battery voltage as well as capacity. On the other hand, parallel will only increase battery capacity.
Welding Lithium Cells
Now, we can join all the cells through spot welding. However, soldering is an alternative way if spot-wielding is not available. However, it generates heat and requires necessary precautions while soldering the cells.
Insulation and BMS Installation
The last step is insulating the battery in a strong battery case and a battery management system (BMS) that performs several important functions. It protects the battery from deep discharge and overcharge and balances the lithium cells.
5부. 자주 묻는 질문
-
Can lithium-ion batteries explode?
A lithium battery can rarely explode. It can overheat and explode if damaged, improperly used, or made with low-quality materials. The safety measures taken during assembly (like spot welding and incorporating a Battery Management System) are crucial to prevent battery explosion or failure. -
How long do lithium-ion batteries last?
The lifespan of a lithium battery can depend on several factors, such as battery type and quality, habit, temperature, and maintenance. However, a LiFePO4 battery has around 2,000 cycles of life. -
How are lithium batteries made?
Making lithium batteries involves several stages. It requires experts and machines to handle different tasks. It includes electrode creation, coating, dying process, and sliting, sealing, forming, and testing stages. -
How can I achieve different voltages and capacities with lithium-ion batteries?
An individual lithium cell typically has 3.7 volts. If you connect these cells in series, it will multiply the volts and capacity. However, suppose you connect the 3.7-volt cell in parallel. In that case, it will only increase the total battery capacity while the voltage will remain at 3.7. -
What is the main mineral in lithium batteries?
A lithium battery uses graphite as an anode of the battery. Cathode can use any of the four minerals, lithium, manganese, cobalt, and nickel. All of these are expensive minerals that make lithium batteries expensive.
관련 태그:
더 많은 기사

The Ultimate Guide to Remove Battery Corrosion
Learn how to remove battery corrosion, prevent future damage, and maintain battery health with our detailed guide.
How to Properly Discard Batteries and Protect the Environment?
Batteries power daily essentials but improper disposal poses risks. Learn to discard them responsibly for sustainable living and environmental protection.
Battery Manufacturing Process: A Comprehensive Guide
The battery manufacturing process creates reliable energy storage units from raw materials, covering material selection, assembly, and testing.
5 Creative Ways to Battery Organiser: Say Goodbye to Clutter
Tired of searching for the right battery? Discover five creative ways to organize batteries and keep your home clutter-free with this efficient storage guide.
Everything You Need to Know About 3.7v Battery
Discover the ins and outs of 3.7V batteries, including types, capacities, and applications. Learn how to choose the best one for your needs.